Problem 1PE: Practice Exercise 9.1 Label the shapes of the following molecules: Problem 2PE: Practice Exercise 9.2 What is the shape of the SeF6 molecule? (Hint: If necessary, refer to Figure... Problem 3PE: Practice Exercise 9.3
What shape is expected for the molecule?
Problem 4PE: Practice Exercise 9.4 The first known compound of the noble gas argon is HArF. What shape is... Problem 5PE: Practice Exercise 9.5
What shape is expected for the ion?
Problem 6PE: Practice Exercise 9.6 What shape is expected for the SO3 molecule? Problem 7PE: Practice Exercise 9.7 Is the sulfur tetrafluoride molecule polar or nonpolar? Explain your... Problem 8PE: Practice Exercise 9.8 Explain how you decided which of The following molecules are expected to be... Problem 9PE: Practice Exercise 9.9 Use the principles of VB theory to explain the bonding in HCl. Give the... Problem 10PE: Practice Exercise 9.10 The phosphine molecule, PH), has a trigonal pyramidal shape with H PH bond... Problem 11PE: Practice Exercise 9.11
The molecule has a planar triangular shape. What kind of hybrid orbitals... Problem 12PE: Practice Exercise 9.12 In the gas phase, beryllium fluoride exists as linear molecules. Which kind... Problem 13PE: Practice Exercise 9.13
What kind of hybrid orbitals arc expected to be used by the central atom in ?... Problem 14PE: What kind of hybrid orbitals are expected to be used by the central atom in PCl5? (Hint: Which... Problem 15PE: Use the VSEPR model to predict the shape of the AsCl5 molecule and then describe the bonding in the... Problem 16PE: What kind of orbitals arc used by Xe in the XeF4 molecule? Give a step-by-step description of how... Problem 17PE: Explain how to decide what kind of hybrid orbitals we would expect the central atom to use for... Problem 18PE: If we assume that nitrogen uses sp3 hybrid orbitals in NH3. use valence bond theory to account for... Problem 19PE: Practice Exercise 9.19
What is the shape of the ion? What hybrid orbitals are used by phosphorus in... Problem 20PE: Practice Exercise 9.20
Consider the molecule below. What kind of hybrid orbitals arc used by atoms... Problem 21PE: Practice Exercise 9.21
Consider the molecule below. What kind of hybrid orbitals arc used by atoms... Problem 22PE: The molecular orbital energy level diagram for the cyanide ion, CN-, is similar to that of the... Problem 23PE: The MO energy level diagram for the nitrogen monoxide molecule is essentially the same as that shown... Problem 24PE: Practice Exercise 9.24
The nitrate ion, , has three resonance structures. The electrons are... Problem 25PE Problem 26PE: Arrange the following elements in order of increasing hand gap: germanium, sulfur, and magnesium. Problem 27PE: Practice Exercise 9.27
What is the hybridization of the carbon atom in (a) diamond, (b) graphite,... Problem 1RQ: Sketch the following molecular shapes and give the various bond angles in the structures: (a) planar... Problem 2RQ: Sketch the following molecular shapes and give the bond angles in the structures: (a) linear, (b)... Problem 3RQ: 9.3 What is the underlying principle on which the VSEPR model is based?
Problem 4RQ: What is an electron domain? How are nonbonding and double bonds described by electron domains? Problem 5RQ: 9.5 How many bonding domains and how many nonbonding domains are there in a molecule of... Problem 6RQ: Sketch the following molecular shapes and give the various bond angles in the structure: (a)... Problem 7RQ: What arrangements of domains around an atom are expected when there are (a) three domains, (b) six... Problem 8RQ: Why is it useful to know the polarities of molecules? Problem 9RQ Problem 10RQ: 9.10 Under what conditions will a molecule be polar
Problem 11RQ: What condition must be met if a molecule having polar bonds is to be nonpolar? Problem 12RQ: Use a drawing to show why the SO2 molecule is polar. Problem 13RQ: What is meant by orbital overlap? Problem 14RQ: How is orbital overlap related to bond energy? Problem 15RQ: Use sketches of orbitals to describe how VB theory would explain the formation of the HBr bond in... Problem 16RQ: 9.16 Why do atoms usually use hybrid orbitals for bonding rather than pure atomic orbitals?
Problem 17RQ: 9.17 Sketch figures that illustrate the directional properties of the following hybrid orbitals: (a)... Problem 18RQ: 9.18 Sketch figures that illustrate the directional properties of (a) , (b) .
Problem 19RQ: 9.19 Why do Period 2 elements never use hybrid orbitals for bond formation?
Problem 20RQ: What relationship is there, if any, between Lewis structures and the valence bond descriptions of... Problem 21RQ: How can the VSEPR model be used to predict the hybridization of an atom in a molecule? Problem 22RQ: If the central oxygen in the water molecule did not use sp3 hybridized orbitals (or orbitals of any... Problem 23RQ: Using orbital diagrams, describe how sp3 hybridization occurs in each atom: (a) carbon, (b)... Problem 24RQ: Sketch the way the orbitals overlap to form the bonds in each of the following: (a) CH4, (b) NH3,... Problem 25RQ: We explained the bond angles of 107inNH3 by using sp3 hybridization of the central nitrogen atom. If... Problem 26RQ: Using sketches of orbitals and orbital diagrams, describe sp2 hybridization of (a) boron and (b)... Problem 27RQ: What two basic shapes have hybridizations that include d orbitals? Problem 28RQ: 9.28 The ammonia molecule, , can combine with a hydrogen ion, (which has an empty 1s orbital), to... Problem 29RQ: 9.29 How does the geometry around B and O change in the following reaction? How does the... Problem 30RQ: How do and bonds differ? Problem 31RQ: Why can free rotation occur easily around a -bond axis but nor around a -bond axis? Problem 32RQ: 9.32 Using sketches, describe the bonds and bond angles in ethene, .
Problem 33RQ: Sketch the way the bonds form in acetylene, C2H2. Problem 34RQ: How does VB theory treat the benzene molecule? (Draw sketches describing the orbital overlaps and... Problem 35RQ: Why is the higher-energy MO in H2 called an antibonding orbital? Make a sketch of the bonding and... Problem 36RQ: Below is an illustration showing two 3d. orbitals about to overlap. The drawings also show the... Problem 37RQ: 9.37 Will the combination of 3d. orbitals in Question 9.36 yield a type of MO? Explain.
Problem 38RQ: Explain why He2 does nor exist but H2 does. Problem 39RQ: 9.39 How does MO theory account for the paramagnetism of ?
Problem 40RQ: 9.40 On the basis of MO theory, explain why molecules can exist but molecules cannot. Could the... Problem 41RQ: 9.41 What relationship is there between bond order and bond energy?
Problem 42RQ: Sketch the shapes of the 2p,and*2p,MOs. Problem 43RQ: 9.43 What is the theoretical basis of both valence bond (VB) theory and molecular orbital (MO)... Problem 44RQ: What shortcomings of Lewis structures and VSEPR theory do VB and MO theories attempt to overcome? Problem 45RQ: What is the main difference in the way VB and MO theories view the bonds in a molecule? Problem 46RQ: What is a delocalized MO? Explain, in terms of orbital overlap, why delocalized MOs are able to form... Problem 47RQ: 9.47 What effect does delocalization have on the stability of the electronic structure of a... Problem 48RQ Problem 49RQ Problem 50RQ: 9.50 What is required to form a conduction band?
Problem 51RQ Problem 52RQ Problem 53RQ: In calcium, why cant electrical conduction take place by movement of electrons through the 2s energy... Problem 54RQ: 9.54 What are allotropes? How do they differ from isotopes?
Problem 55RQ: Why are the Period 2 elements able to form much stronger bonds than the non metals of Period 3? Why... Problem 56RQ: Even though the nonmetals of Periods 3, 4, and 5 do not tend to form bonds between like atoms, each... Problem 57RQ: Which of the nonmetals occur in nature in the form of isolated atoms? Problem 58RQ: 9.58 Describe the structure of diamond. What kind of hybrid orbitals does carbon use to form bonds... Problem 59RQ: Describe the structure of graphene. What kind of hybrid orbitals does carbon use in the formation of... Problem 60RQ: How is the structure of graphite related to the structure of graphene? Problem 61RQ Problem 62RQ: 9.62 How is the structure of a carbon nanotube related to the structure of graphene?
Problem 63RQ: 9.63 What is the molecular structure of silicon? Suggest a reason why silicon doesn't form an... Problem 64RQ: Make a sketch that describes the molecular structure of white phosphorus. Problem 65RQ: 9.65 What are the different allotropes of phosphorus?
Problem 66RQ: 9.66 What are the P—P—P bond angles in the molecule? If phosphorus uses p orbitals to form the... Problem 67RQ Problem 68RQ: 9.68 What is the molecular structure of black phosphorus? In what way does the structure of black... Problem 69RQ: What are the two allotropes of oxygen? Problem 70RQ: Draw the Lewis structure for O3. Is the molecule linear, based on the VSEPR model? Assign formal... Problem 71RQ: 9.71 What beneficial function does ozone serve in earths upper atmosphere?
Problem 72RQ: What is the molecular structure of sulfur in its most stable allotropic form? Problem 73RQ: 9.73 Predict the shapes of (a) , (b) , (c) , (d) , and (e) .
Problem 74RQ: Predict the shapes of (a) SF3+, (b) GeF4, (c) , (d) O3, and (e) N2O. Problem 75RQ: Predict the shapes of (a)FC12+,(b)AsF5,(c)AsF3,(d)SbH3,and(e)SeO2. Problem 76RQ: Predict the shapes of (a) TeF4, (b) SbCl6, (c) NO2, (d) PCl4, and (e) PO43-. Problem 77RQ: Predict the shapes of (a)IO4,(b)IF4,(c)TeF6,(d)SiO4and(e)IC12. Problem 78RQ: 9.78 Predict the shapes of .
Problem 79RQ: Which of the following has a shape described by the figure below:... Problem 80RQ: Which of the following has a shape described by the figure below: (a)BrF3,(b)PF3,(c)NO3,or(d)SCl3? Problem 81RQ: Ethene, also called ethylene, is a gas used to ripen tomatoes artificially. It has the Lewis... Problem 82RQ: Ethyne, more commonly called acetylene, is a gas used in welding torches. It has the Lewis structure... Problem 83RQ: 9.83 Predict the bond angle for each of the following molecules:
Problem 84RQ: 9.84 Predict the bond angle for each of the following ions:
Problem 85RQ: 9.85 Which of the following molecules would be expected to be polar?
Problem 86RQ: Which of the following molecules would he expected to be polar?... Problem 87RQ: Which of the following molecules or ions would be expected to have a net dipole moment?... Problem 88RQ: Which of the following molecules or ions would be expected to have a net dipole moment?... Problem 89RQ: 9.89 Explain why is nonpolar, but is polar.
Problem 90RQ: 9.90 Explain why is polar, but is not.
Problem 91RQ: Use sketches of orbitals to show how VB theory explains the bonding in the Cl2 molecule. Illustrate... Problem 92RQ: Hydrogen selenide is one of nature's most foul-smelling substances. Molecules of H2Se have HSeH bond... Problem 93RQ: Use orbital diagrams to explain how the beryllium chloride molecule is formed. What kind of hybrid... Problem 94RQ: Use orbital diagrams to describe the bonding in tin tetrachloride? What kind of hybrid orbitals does... Problem 95RQ: 9.95 Use orbital diagrams to describe the bonding in antimony pentachloride, a substance used to add... Problem 96RQ: Describe the bonding in tellurium hexafluoride, a toxic gas with an extremely unpleasant smell.... Problem 97RQ: Draw Lewis structures for the following and use the geometry predicted by the VSEPR model to... Problem 98RQ: Draw Lewis structures for the following and use the geometry predicted by the VSEPR model to... Problem 99RQ: Use the VSEPR model to help you describe the bonding in the following molecules according to VB... Problem 100RQ: Use the VSEPR model to help you describe the bonding in the following molecules according to VB... Problem 101RQ: 9.101 Use orbital diagrams to show that the bonding in involves the formation of a coordinate... Problem 102RQ: What kind of hybrid orbitals are used by tin in SnCl62? Draw the orbital diagram for Sn in SnCl62.... Problem 103RQ: A nitrogen atom can undergo sp2 hybridization when it becomes part of a carbon-nitrogen double bond,... Problem 104RQ: A nitrogen atom can undergo sp hybridization and then become joined to carbon by a triple bond. (a)... Problem 105RQ: Tetrachloroethylene, a common dry-cleaning solvent, has the formula C2Cl4. Its structure is Use the... Problem 106RQ: 9.106 Phosgene, , was used as a war gas during World War I. It reacts with moisture in the lungs of... Problem 107RQ: 9.107 What kind of hybrid orbitals do the numbered atoms use in the following molecule?
Problem 108RQ: What kind of hybrid orbitals do the numbered atoms use in the following molecule? Problem 109RQ: 9.109 What kinds of bonds are found in the numbered bonds in the following molecule?
Problem 110RQ: 9.110 What kinds of bondsare found in the numbered bonds in the following molecule?
Problem 111RQ: Construct the molecular orbital diagram for O2. What is the net bond order in O2? Problem 112RQ: Construct the molecular orbital diagram for N2. What is the new bond order in N2? Problem 113RQ: Use the MO energy diagram to predict (a) the bond orders for each molecule or ion, (b) which one has... Problem 114RQ: Use the MO energy diagram to predict (a) the bond orders for each molecule or ion, (b) which one has... Problem 115RQ: Assume that in the NO molecule the molecular orbital energy level sequence is similar to that for... Problem 116RQ: 9.116 Assume that in the NO molecule the molecular orbital energy level sequence is similar to that... Problem 117RQ: Which of the following molecules or ions are paramagnetic? (a)O2+,(b)O2,(c)O2,(d)NO,(e)N2 Problem 118RQ: 9.118 Which of the following molecules or ions are paramagnetic?
Problem 119RQ: *9.119 Construct the MO energy level diagram for the OH molecule assuming it is similar to that for... Problem 120RQ: If boron and nitrogen were to form a molecule with the formula BN, what would its MO energy level... Problem 121RQ: 9.121 Formaldehyde has the Lewis structure
What would you predict its shape to be?
Problem 122RQ Problem 123RQ: Antimony forms a compound with hydrogen that is called stibine. Its formula is SbH3 and the H Sb H... Problem 124RQ: Describe the changes in molecular geometry and hybridization that take place during the following... Problem 125RQ Problem 126RQ Problem 127RQ: Phosphorus trifluoride, PF3, has FPF bond angles of 97.8. (a) How would VB theory use hybrid... Problem 128RQ: A six-membered ring of carbons can hold a double bond but not a triple bond. Explain. Problem 129RQ: The more electronegative are the atoms bonded to the central atom, the less are the repulsions... Problem 130RQ: Alone pair of electrons in the valence shell of an atom has a larger effective volume than a bonding... Problem 131RQ: *9.131 The two electron pairs in a double bond repel other electron pairs more than the single pair... Problem 132RQ: In a certain molecule, ap orbital overlaps with a d orbital as shown. The algebraic signs of the... Problem 133RQ: *9.133 If we assign the internuclear axis in a diatomic molecule to be the z axis, what kind of p... Problem 134RQ: The peroxynitrite ion, OONO-, is a potent toxin formed in cells affected by diseases such as... Problem 135RQ: *9.135 An ammonia molecule, , is very polar, whereas is almost nonpolar. Use this observation along... Problem 136RQ: There exists a hydrocarbon called butadiene, which has the molecular formula C4H6 and the structure... Problem 137RQ Problem 138RQ: 9.138 Five basic molecular shapes were described for simple molecular structures containing a... Problem 139RQ: 9.139 Compare and contrast the concepts of delocalization and resonance.
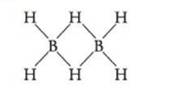
Problem 140RQ: Why doesnt a carbon-carbon quadruple bond exist? Problem 141RQ: What might the structure of the iodine heptafluoride molecule be? If you can think of more than one... Problem 142RQ: The FF bond in F2 is weaker than the ClCl bond in Cl2. How might the lone pairs on the atoms in the... Problem 143RQ: Molecular orbital theory predicts the existence of anti- bonding molecular orbitals. How do... Problem 144RQ: The structure of the diborane molecule, B2H6, is sometimes drawn as There are not enough valence... format_list_bulleted


 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning




