
Concept explainers
a)
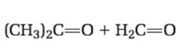
Interpretation:
The structure of the alkene that yields (CH3)2C=O + H2C=O as products when treated with ozone followed by treatment with Zn is to be given.
Concept introduction:
Ozone adds to the double bond in
To give:
The structure of the alkene that yields (CH3)2C=O + H2C=O as products when treated with ozone followed by treatment with Zn.
b)
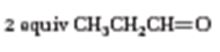
Interpretation:
The structure of the alkene that yields 2 equivalents of CH3CH2CH=O as products when treated with ozone followed by treatment with Zn is to be given.
Concept introduction:
Ozone adds to the double bond in alkenes to produce compounds called ozonides. The ozonides upon immediate treatment with Zn and acetic acid yield carbonyl compounds. Each carbon in the double bond cleaved gets attached to an oxygen atom. Hence the alkene should contain the carbons that contain oxygen atom in the products in a doubly bond.
To give:
The structure of the alkene that yields 2 equivalents of CH3CH2CH=O as products when treated with ozone followed by treatment with Zn.
Trending nowThis is a popular solution!

Chapter 8 Solutions
Organic Chemistry
- The reaction Q(g) + R(g) → Z(l) is shown to be exothermic. Which of the following is true concerning the reactionarrow_forwardWhich of the following has the largest standard molar entropy, S° (298.15 K) He H2 NaCl KBr Hgarrow_forwardWhich of the following is true for a particular reaction if ∆G° is -40.0 kJ/mol at 290 K and –20.0 kJ/mol at 390 K?arrow_forward
- Choose the major product of the reaction with correct regio- and stereochemistry. Br2 H₂O O "Br Br & O 'Br OH Br 吡 O OH OH Br "OH Brarrow_forwardSelect the major product of the following reaction. & Br (CH)CONa (CH₂),COH 0 OC(CH) O &arrow_forwardDraw the products of the hydrolysis reaction between the ester molecule and water. Determine the products of the following reaction.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

