
(a)
Interpretation:
Whether the given compound is homotopic, enantiotopic or diastereotopic needs to be predicted and the chemical shifts in ( and its splitting patterns based on the hydrogen present needs to be determined, assuming that the coupling constant of all the neighboring atoms is identical.
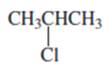
Concept Introduction :
Compound is homotopic, if molecule has same elements which when interchanged is the same. Compound is enantiotopic, if the two groups in the molecule when replaced generates achiral compound and the resulting products formed are enantiomers. Compund is diasterotopic if the two groups in molecule when replaced would generate compounds which are diastereomers.
(b)
Interpretation:
Whether the given compound is homotopic, enantiotopic or diastereotopic needs to be predicted and the chemical shifts in ( and its splitting patterns based on the hydrogen present needs to be determined, assuming that the coupling constant of all the neighboring atoms is identical.
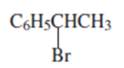
Concept Introduction :
Compound is homotopic, if molecule has same elements which when interchanged is the same. Compound is enantiotopic, if the two groups in the molecule when replaced generates achiral compound and the resulting products formed are enantiomers. Compund is diasterotopic if the two groups in molecule when replaced would generate compounds which are diastereomers.
(c)
Interpretation:
Whether the given compound is homotopic, enantiotopic or diastereotopic needs to be predicted and the chemical shifts in ( and its splitting patterns based on the hydrogen present needs to be determined, assuming that the coupling constant of all the neighboring atoms is identical.
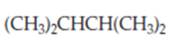
Concept Introduction :
Compound is homotopic, if molecule has same elements which when interchanged is the same. Compound is enantiotopic, if the two groups in the molecule when replaced generates achiral compound and the resulting products formed are enantiomers. Compund is diasterotopic if the two groups in molecule when replaced would generate compounds which are diastereomers.
(d)
Interpretation:
Whether the given compound is homotopic, enantiotopic or diastereotopic needs to be predicted and the chemical shifts in ( and its splitting patterns based on the hydrogen present needs to be determined, assuming that the coupling constant of all the neighboring atoms is identical.
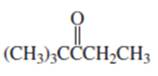
Concept Introduction :
Compound is homotopic, if molecule has same elements which when interchanged is the same. Compound is enantiotopic, if the two groups in the molecule when replaced generates achiral compound and the resulting products formed are enantiomers. Compund is diasterotopic if the two groups in molecule when replaced would generate compounds which are diastereomers.
(e)
Interpretation:
Whether the given compound is homotopic, enantiotopic or diastereotopic needs to be predicted and the chemical shifts in ( and its splitting patterns based on the hydrogen present needs to be determined, assuming that the coupling constant of all the neighboring atoms is identical.
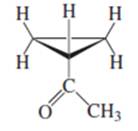
Concept Introduction :
Compound is homotopic, if molecule has same elements which when interchanged is the same. Compound is enantiotopic, if the two groups in the molecule when replaced generates achiral compound and the resulting products formed are enantiomers. Compound is diasterotopic if the two groups in molecule when replaced would generate compounds which are diastereomers.
(f)
Interpretation:
Whether the given compound is homotopic, enantiotopic or diastereotopic needs to be predicted and the chemical shifts in ( and its splitting patterns based on the hydrogen present needs to be determined, assuming that the coupling constant of all the neighboring atoms is identical.
Concept Introduction :
Compound is homotopic, if molecule has same elements which when interchanged is the same. Compound is enantiotopic, if the two groups in the molecule when replaced generates achiral compound and the resulting products formed are enantiomers. Compound is diasterotopic if the two groups in molecule when replaced would generate compounds which are diastereomers.
(g)
Interpretation:
Whether the given compound is homotopic, enantiotopic or diastereotopic needs to be predicted and the chemical shifts in ( and its splitting patterns based on the hydrogen present needs to be determined, assuming that the coupling constant of all the neighboring atoms is identical.
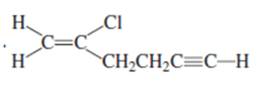
Concept Introduction :
Compound is homotopic, if molecule has same elements which when interchanged is the same. Compound is enantiotopic, if the two groups in the molecule when replaced generates achiral compound and the resulting products formed are enantiomers. Compound is diasterotopic if the two groups in molecule when replaced would generate compounds which are diastereomers.
(h)
Interpretation:
Whether the given compound is homotopic, enantiotopic or diastereotopic needs to be predicted and the chemical shifts in ( and its splitting patterns based on the hydrogen present needs to be determined, assuming that the coupling constant of all the neighboring atoms is identical.
Concept Introduction :
Compound is homotopic, if molecule has same elements which when interchanged is the same. Compound is enantiotopic, if the two groups in the molecule when replaced generates achiral compound and the resulting products formed are enantiomers. Compund is diasterotopic if the two groups in molecule when replaced would generate compounds which are diastereomers.
(i)
Interpretation:
Whether the given compound is homotopic, enantiotopic or diastereotopic needs to be predicted and the chemical shifts in ( and its splitting patterns based on the hydrogen present needs to be determined, assuming that the coupling constant of all the neighboring atoms is identical.
Concept Introduction :
Compound is homotopic, if molecule has same elements which when interchanged is the same. Compound is enantiotopic, if the two groups in the molecule when replaced generates achiral compound and the resulting products formed are enantiomers. Compund is diasterotopic if the two groups in molecule when replaced would generate compounds which are diastereomers.
(j)
Interpretation:
Whether the given compound is homotopic, enantiotopic or diastereotopic needs to be predicted and the chemical shifts in ( and its splitting patterns based on the hydrogen present needs to be determined, assuming that the coupling constant of all the neighboring atoms is identical.
Concept Introduction :
Compound is homotopic, if molecule has same elements which when interchanged is the same. Compound is enantiotopic, if the two groups in the molecule when replaced generates achiral compound and the resulting products formed are enantiomers. Compund is diasterotopic if the two groups in molecule when replaced would generate compounds which are diastereomers.
Want to see the full answer?
Check out a sample textbook solution
Chapter 8 Solutions
Experimental Organic Chemistry: A Miniscale & Microscale Approach (Cengage Learning Laboratory Series for Organic Chemistry)
- 2-Propanone and ethyllithium are mixed and subsequently acid hydrolyzed. Draw and name the structures of the products.arrow_forward(Methanesulfinyl)methane is reacted with NaH, and then with acetophenone. Draw and name the structures of the products.arrow_forward3-Oxo-butanenitrile and (E)-2-butenal are mixed with sodium ethoxide in ethanol. Draw and name the structures of the products.arrow_forward
- What is the reason of the following(use equations if possible) a.) In MO preperation through diazotization: Addition of sodium nitrite in acidfied solution in order to form diazonium salt b.) in MO experiment: addition of sodium hydroxide solution in the last step to isolate the product MO. What is the color of MO at low pH c.) In MO experiment: addition of sodium hydroxide solution in the last step to isolate the product MO. What is the color of MO at pH 4.5 d.) Avoiding not cooling down the reaction mixture when preparing the diazonium salt e.) Cbvcarrow_forwardA 0.552-g sample of an unknown acid was dissolved in water to a total volume of 20.0 mL. This sample was titrated with 0.1103 M KOH. The equivalence point occurred at 29.42 mL base added. The pH of the solution at 10.0 mL base added was 3.72. Determine the molar mass of the acid. Determine the Ka of the acid.arrow_forwardAs the lead product manager at OrganometALEKS Industries, you are trying to decide if the following reaction will its major product: 2,0° with a new C-C bond as If this reaction will work, draw the major organic product or products you would expect in the drawing aree below. If there's more than one major product, you can draw them in any arrangement you like. Be sure you use wedge and desh bonds if necessary, for example to distinguish between major products with different stereochemistry. If the major products of this reaction won't have a new C-C bond, just check the box under the drawing area and leave it blank.arrow_forward
- write the mechanism of the nucleophilic acyl substitution reaction, please give an examplearrow_forwardThe compound in the figure is reacted with 10 n-butyllihium, 2° propanone, and 3º H2O. Draw and name the products obtained. SiMe3arrow_forwardCaffeine (C8H10N4O2, pictured below) is a weak base. The pKb of caffeine is 10.4. What is the pH of a 0.0155 M solution of caffeine?arrow_forward
- 2-Cyclopentyl-2-methyl-1,3-dioxolane is reacted with H₂SO₄. Draw and name the structures of the products.arrow_forwardIndicate the products of the reaction of 1-cyclohexyl-2,2-dimethylpropan-1-one with CH3CO3H (). Draw the structures of the compounds.arrow_forwardWrite chemical equations for: the reaction of benzoic acid chloride with grignard reagent [CH3MgX] the reaction of butanoic acid with methyl amine [CH3NH2]arrow_forward
 Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,



