
Elements Of Physical Chemistry
7th Edition
ISBN: 9780198727873
Author: ATKINS, P. W. (peter William), De Paula, Julio
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Question
Chapter 8, Problem 8B.4ST
(a)
Interpretation Introduction
Interpretation:
The ground state electronic configuration of
Concept-Introduction:
Aufbau principle:
- Electrons first occupy orbitals with lower energy than higher energy.
- An orbital can be occupied only by two electrons having opposite spins.
- Each electron fills each orbital till it is half filled. Greater the number of parallel spins of electrons more stable the orbital. This is known as Hund’s rule.
- The order of electrons filling in an atom is given as
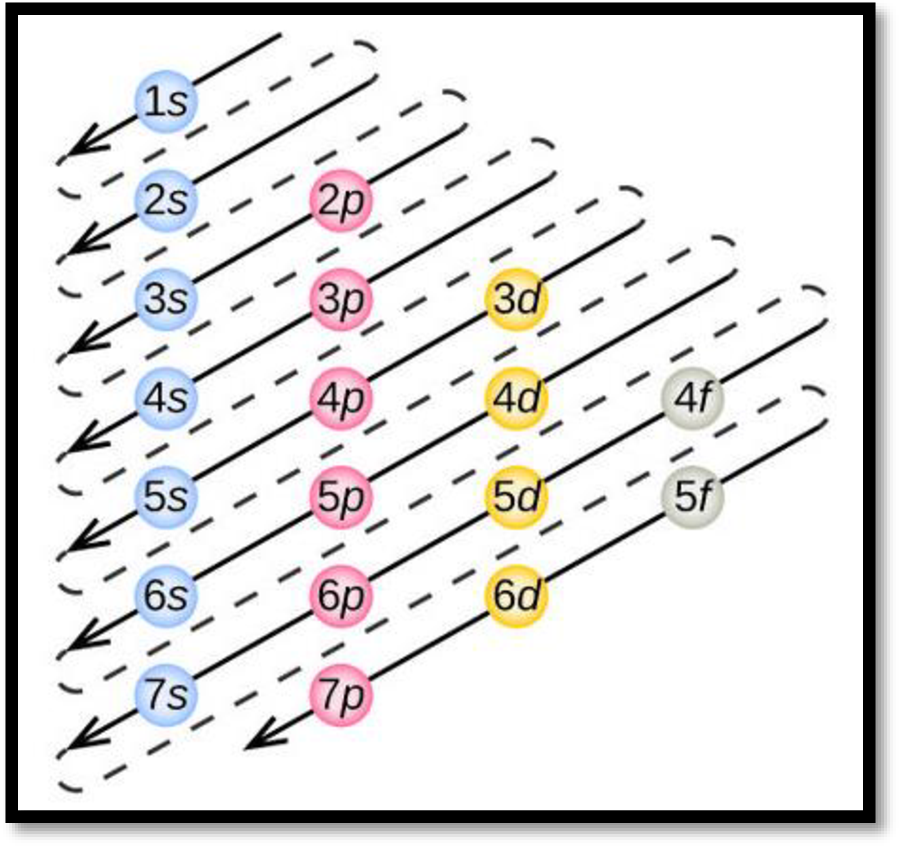
Figure 1
(b)
Interpretation Introduction
Interpretation:
The ground state electronic configuration of
Concept-Introduction:
Aufbau principle:
- Electrons first occupy orbitals with lower energy than higher energy.
- An orbital can be occupied only by two electrons having opposite spins.
- Each electron fills each orbital till it is half filled. Greater the number of parallel spins of electrons more stable the orbital. This is known as Hund’s rule.
- The order of electrons filling in an atom is given as
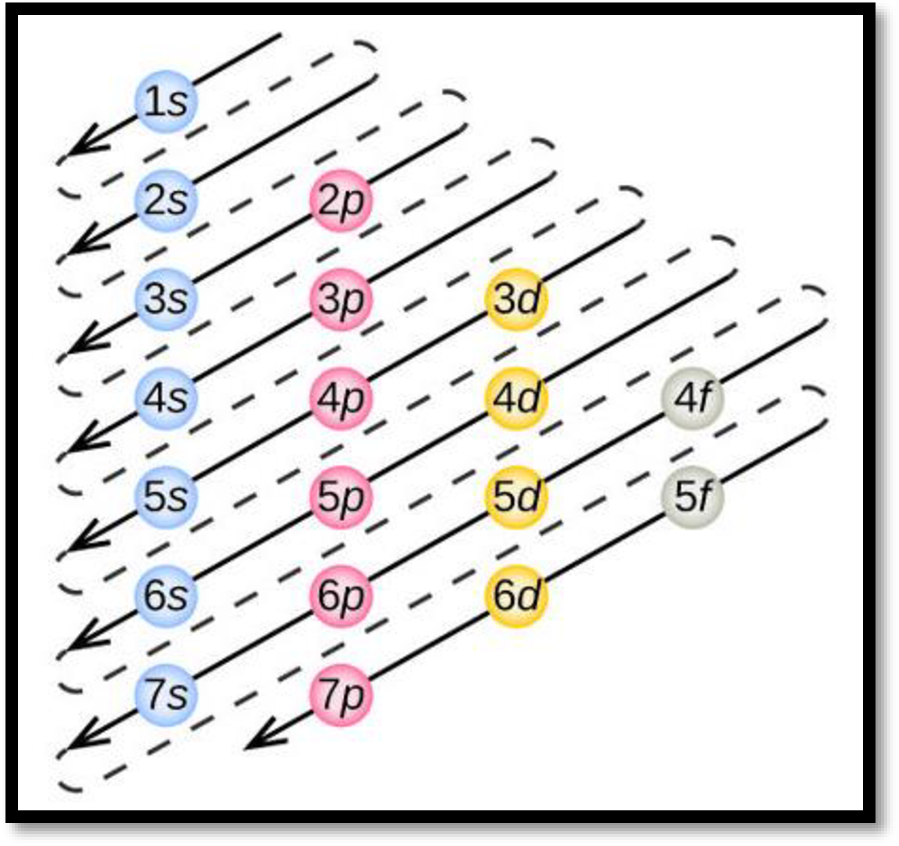
Figure 1
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Blocking Group are use to put 2 large sterically repulsive group ortho. Show the correct sequence toconnect the reagent to product with the highest yield possible. * see image **NOTE: The compound on the left is the starting point, and the compound on the right is the final product. Please show the steps in between to get from start to final, please. These are not two different compounds that need to be worked.
I dont understand this.
Can you please explain this prooblem to me, show me how the conjugation is added, did I add them in the correct places and if so please show me. Thanks!
Chapter 8 Solutions
Elements Of Physical Chemistry
Ch. 8 - Prob. 8A.1STCh. 8 - Prob. 8A.2STCh. 8 - Prob. 8A.3STCh. 8 - Prob. 8A.4STCh. 8 - Prob. 8A.5STCh. 8 - Prob. 8B.2STCh. 8 - Prob. 8B.3STCh. 8 - Prob. 8B.4STCh. 8 - Prob. 8C.1STCh. 8 - Prob. 8C.2ST
Ch. 8 - Prob. 8D.1STCh. 8 - Prob. 8D.2STCh. 8 - Prob. 8D.3STCh. 8 - Prob. 8D.4STCh. 8 - Prob. 8D.5STCh. 8 - Prob. 8D.6STCh. 8 - Prob. 8D.7STCh. 8 - Prob. 8A.1ECh. 8 - Prob. 8A.2ECh. 8 - Prob. 8A.3ECh. 8 - Prob. 8A.6ECh. 8 - Prob. 8A.7ECh. 8 - Prob. 8A.8ECh. 8 - Prob. 8A.9ECh. 8 - Prob. 8A.10ECh. 8 - Prob. 8A.12ECh. 8 - Prob. 8B.1ECh. 8 - Prob. 8B.2ECh. 8 - Prob. 8B.3ECh. 8 - Prob. 8C.1ECh. 8 - Prob. 8C.2ECh. 8 - Prob. 8D.1ECh. 8 - Prob. 8D.2ECh. 8 - Prob. 8D.3ECh. 8 - Prob. 8D.4ECh. 8 - Prob. 8D.5ECh. 8 - Prob. 8D.6ECh. 8 - Prob. 8D.7ECh. 8 - Prob. 8D.8ECh. 8 - Prob. 8D.9ECh. 8 - Prob. 8D.11ECh. 8 - Prob. 8D.12ECh. 8 - Prob. 8.1DQCh. 8 - Prob. 8.2DQCh. 8 - Prob. 8.3DQCh. 8 - Prob. 8.4DQCh. 8 - Prob. 8.5DQCh. 8 - Prob. 8.6DQCh. 8 - Prob. 8.7DQCh. 8 - Prob. 8.8DQCh. 8 - Prob. 8.2PCh. 8 - Prob. 8.4PCh. 8 - Prob. 8.7PCh. 8 - Prob. 8.8PCh. 8 - Prob. 8.9PCh. 8 - Prob. 8.11PCh. 8 - Prob. 8.12P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Basic strength of organic bases.arrow_forwardNucleophilic Aromatic Substitution: What is the product of the reaction? What is the name of the intermediate complex? *See imagearrow_forwardPredict the final product. If 2 products are made, list which should be “major” and “minor” *see attachedarrow_forward
- Nucleophilic Aromatic Substitution: What is the product of the reaction? *see imagearrow_forwardShow the correct sequence to connect the reagent to product. * see imagearrow_forwardThe answer here says that F and K have a singlet and a doublet. The singlet and doublet are referring to the H's 1 carbon away from the carbon attached to the OH. Why don't the H's two carbons away, the ones on the cyclohexane ring, cause more peaks on the signal?arrow_forward
- Draw the Birch Reduction for this aromatic compound and include electron withdrawing groups and electron donating groups. *See attachedarrow_forwardShow the correct sequence to connect the reagent to product. * see imagearrow_forwardBlocking Group are use to put 2 large sterically repulsive group ortho. Show the correct sequence toconnect the reagent to product with the highest yield possible. * see imagearrow_forward
- Elimination-Addition: What molecule was determined to be an intermediate based on a “trapping experiment”? *please solve and see imagearrow_forwardShow the correct sequence to connect the reagent to product. * see imagearrow_forwardPredict the final product. If 2 products are made, list which should be “major” and “minor”. **see attachedarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Introduction to Coordination ComplexesWave Function for Hydrogen atom # All Vital Topics # Quantum Mechanics part -21; Author: Priyanka Jain;https://www.youtube.com/watch?v=GKgNV9dmUHo;License: Standard YouTube License, CC-BY
CBSE Class 12 Chemistry || The d & f Block Elements Part 1 || Full Chapter || By Shiksha House; Author: Best for NEET;https://www.youtube.com/watch?v=LzZWHSdYaxw;License: Standard Youtube License