
Concept explainers
Interpretation: The preferred resonance form among the given ions is to be stated on the basis of formal charges.
Concept introduction: Formal charges play an important role in choosing between the possible molecular structures. The preferred structure is the one in which formal charges are zero.
To determine: The preferred resonance form among the given ions on the basis of formal charges.
Answer to Problem 8.92QP
Solution
The preferred resonance form in the
The preferred resonance form in the
The preferred resonance form in the
Explanation of Solution
Explanation
The given ion is
Therefore the total valence electrons are
The lone pair of electrons on oxygen and bonding electrons is delocalized which results in the formation of lewis structures. The lewis structures for

Figure 1
The formal charge on each atom of resonating structure (a) of
Formal charge is calculated as,
Number of valence electrons in nitrogen is
Number of lone pair electrons in nitrogen is
Number of bond pair electrons in nitrogen is
To calculate the formal charge on nitrogen, substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
Number of valence electrons in oxygen is
Number of lone pair electrons in oxygen is
Number of bond pair electrons in oxygen is
To calculate the formal charge on oxygen, substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
Number of valence electrons in carbon is
Number of lone pair electrons in carbon is
Number of bond pair electrons in carbon is
To calculate the formal charge on carbon, substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
The formal charge on each atom of resonating structure (b) of
Number of valence electrons in nitrogen is
Number of lone pair electrons in nitrogen is
Number of bond pair electrons in nitrogen is
To calculate the formal charge on nitrogen, substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
Number of valence electrons in oxygen is
Number of lone pair electrons in oxygen is
Number of bond pair electrons in oxygen is
To calculate the formal charge on oxygen, substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
Number of valence electrons in carbon is
Number of lone pair electrons in carbon is
Number of bond pair electrons in carbon is
To calculate the formal charge on carbon, substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
According to formal charge calculations, there is a formal charge of
The given ion is
Therefore the total valence electrons are
Since carbon is least electronegative, it will act as central atom. The lone pair of electrons on oxygen and bonding electrons is delocalized which results in the formation of lewis structures. The lewis structures for
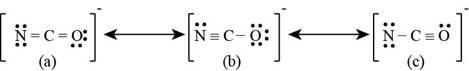
Figure 2
The formal charge on each atom of resonating structure (a) of
Number of valence electrons in nitrogen is
Number of lone pair electrons in nitrogen is
Number of bond pair electrons in nitrogen is
To calculate the formal charge on nitrogen, substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
Number of valence electrons in oxygen is
Number of lone pair electrons in oxygen is
Number of bond pair electrons in oxygen is
To calculate the formal charge on oxygen, substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
Number of valence electrons in carbon is
Number of lone pair electrons in carbon is
Number of bond pair electrons in carbon is
To calculate the formal charge on carbon, substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
The formal charge on each atom of resonating structure (b) of
Number of valence electrons in nitrogen is
Number of lone pair electrons in nitrogen is
Number of bond pair electrons in nitrogen is
To calculate the formal charge on nitrogen, substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
Number of valence electrons in oxygen is
Number of lone pair electrons in oxygen is
Number of bond pair electrons in oxygen is
To calculate the formal charge on oxygen, substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
Number of valence electrons in carbon is
Number of lone pair electrons in carbon is
Number of bond pair electrons in carbon is
To calculate the formal charge on carbon, substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
The formal charge on each atom of resonating structure (c) of
Number of valence electrons in nitrogen is
Number of lone pair electrons in nitrogen is
Number of bond pair electrons in nitrogen is
To calculate the formal charge on nitrogen, substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
Number of valence electrons in oxygen is
Number of lone pair electrons in oxygen is
Number of bond pair electrons in oxygen is
To calculate the formal charge on oxygen, substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
Number of valence electrons in carbon is
Number of lone pair electrons in carbon is
Number of bond pair electrons in carbon is
To calculate the formal charge on carbon, substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
According to formal charge calculations, there is a formal charge of
The given ion is
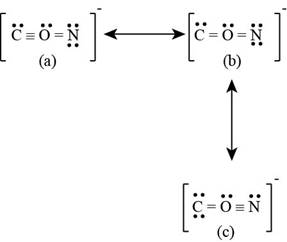
Figure 3
The formal charge on each atom of resonating structure (a) of
Number of valence electrons in nitrogen is
Number of lone pair electrons in nitrogen is
Number of bond pair electrons in nitrogen is
To calculate the formal charge on nitrogen, substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
Number of valence electrons in oxygen is
Number of lone pair electrons in oxygen is
Number of bond pair electrons in oxygen is
To calculate the formal charge on oxygen, substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
Number of valence electrons in carbon is
Number of lone pair electrons in carbon is
Number of bond pair electrons in carbon is
To calculate the formal charge on carbon, substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
The formal charge on each atom of resonating structure (b) of
Number of valence electrons in nitrogen is
Number of lone pair electrons in nitrogen is
Number of bond pair electrons in nitrogen is
To calculate the formal charge on nitrogen, substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
Number of valence electrons in oxygen is
Number of lone pair electrons in oxygen is
Number of bond pair electrons in oxygen is
To calculate the formal charge on oxygen, substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
Number of valence electrons in carbon is
Number of lone pair electrons in carbon is
Number of bond pair electrons in carbon is
To calculate the formal charge on carbon, substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
The formal charge on each atom of resonating structure (c) of
Number of valence electrons in nitrogen is
Number of lone pair electrons in nitrogen is
Number of bond pair electrons in nitrogen is
To calculate the formal charge on nitrogen, substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
Number of valence electrons in oxygen is
Number of lone pair electrons in oxygen is
Number of bond pair electrons in oxygen is
To calculate the formal charge on oxygen, substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
Number of valence electrons in carbon is
Number of lone pair electrons in carbon is
Number of bond pair electrons in carbon is
To calculate the formal charge on carbon, substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
According to formal charge calculations, resonating structure (a) and (c) least electronegative atom carbon possess
Conclusion
The preferred resonance form in the
The preferred resonance form in the
The preferred resonance form in the
Want to see more full solutions like this?
Chapter 8 Solutions
Chemistry: The Science in Context (Fifth Edition)
- Name the major organic product of the following action of 4-chloro-4-methyl-1-pentanol in neutral pollution 10+ Now the product. The product has a molecular formula f b. In a singly hain, the starting, material again converts into a secule with the molecular kormula CIO. but with comply Draw the major organic structure inhalationarrow_forwardMacmillan Learning Alcohols can be oxidized by chromic acid derivatives. One such reagent is pyridinium chlorochromate, (C,H,NH*)(CICTO3), commonly known as PCC. Draw the proposed (neutral) intermediate and the organic product in the oxidation of 1-butanol by PCC when carried out in an anhydrous solvent such as CH₂C₁₂. PCC Intermediate OH CH2Cl2 Draw the intermediate. Select Draw Templates More с H Cr о Product Draw the product. Erase Select Draw Templates More H о Erasearrow_forwardIf I have 1-bromopropene, to obtain compound A, I have to add NaOH and another compound. Indicate which compound that would be. A C6H5 CH3arrow_forward
- Provide the reagents for the following reactions.arrow_forwardIf I have 1-bromopropene, to obtain compound Z, I have to add two compounds A1 and A2. Indicate which compounds are needed. P(C6H5)3arrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. Assume that the water side product is continuously removed to drive the reaction toward products. O CH3CH2NH2, TSOH Select to Draw >arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





