
Package: Loose Leaf for Organic Chemistry with Biological Topics with Connect Access Card
5th Edition
ISBN: 9781260170405
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 8, Problem 8.51P
Draw the structure of a dihalide that could be used to prepare each
a. 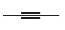 b.
b. 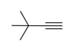 c.
c. 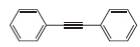
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
d) Determine the formal charge on the nitrogen atom in each of the structures.
NH3
NH2
N
C
бобкат
: N
N
H
H
Н
H2N-OH
A
B
C
D
E
F
G
Lewis Structure, Hybridization & Molecular Geometry
a) Draw the Lewis Structure of the molecules; Label the hybridization of each carbon atom;
Predict the approximate molecular geometry around each carbon atom.
CH3CHO
CH3CN
b) Draw the Lewis Structure of Nitromethane; Predict the approximate molecular geometry
around the nitrogen atom.
CH3NO2
c) Draw the Lewis Structure; Label the hybridization of the boron atom; Predict the
approximate molecular geometry.
BF3
BF4
a. The structure of the bicarbonate (hydrogen carbonate) ion, HCO3-, HCO3 " is
best described as a hybrid of several contributing resonance forms, two of which
are shown here.
HO
:0:
HO
+
:Ö:
Bicarbonate is crucial for the control of body pH (for example, blood pH
7.4). A more self-indulgent use is in baking soda, where it serves as a
source of CO2 CO2 gas, which gives bread and pastry their fluffy
constituency.
(i) Draw at least one additional resonance form.
=
(ii) Using curved "electron-pushing" arrows, show how these Lewis structures may
be interconverted by movement of electron pairs. (iii) Determine which form or
forms will be the major contributor(s) to the real structure of bicarbonate,
explaining your answer on the basis of the criteria in Section 1-5.
Chapter 8 Solutions
Package: Loose Leaf for Organic Chemistry with Biological Topics with Connect Access Card
Ch. 8 - Problem 8.1 Label the and carbons in each alkyl...Ch. 8 - Problem 8.2 Classify each alkene in the following...Ch. 8 - Prob. 8.3PCh. 8 - Prob. 8.4PCh. 8 - Problem 8.5 Label each pair of alkenes as...Ch. 8 - Problem 8.6 Which alkene in each pair is more...Ch. 8 - Problem 8.7 Several factors can affect alkene...Ch. 8 - Prob. 8.8PCh. 8 - Prob. 8.9PCh. 8 - Prob. 8.10P
Ch. 8 - Prob. 8.11PCh. 8 - Problem 8.12 What alkenes are formed from each...Ch. 8 - Prob. 8.13PCh. 8 - Problem 8.14 What alkenes are formed from each...Ch. 8 - Problem 8.15 How does each of the following...Ch. 8 - Problem 8.16 Draw both the SN1 and E1 products of...Ch. 8 - Prob. 8.17PCh. 8 - Prob. 8.18PCh. 8 - Problem 8.19 Explain why...Ch. 8 - Prob. 8.20PCh. 8 - Problem 8.21 Draw the alkynes formed when each...Ch. 8 - Problem 8.22 Draw the products in each...Ch. 8 - Problem 8.23 Draw a stepwise mechanism for the...Ch. 8 - 8.24 Rank the alkenes shown in the ball-and-stick...Ch. 8 - Prob. 8.25PCh. 8 - 8.26 What is the major E2 elimination product...Ch. 8 - Prob. 8.27PCh. 8 - Prob. 8.28PCh. 8 - Prob. 8.29PCh. 8 - 8.30 Label each pair of alkenes as constitutional...Ch. 8 - Prob. 8.31PCh. 8 - Prob. 8.32PCh. 8 - Prob. 8.33PCh. 8 - For each of the following alkenes, draw the...Ch. 8 - Prob. 8.35PCh. 8 - Prob. 8.36PCh. 8 - Prob. 8.37PCh. 8 - What alkene is the major product formed from each...Ch. 8 - Prob. 8.39PCh. 8 - Prob. 8.40PCh. 8 - Pick the reactant or solvent in each part that...Ch. 8 - 8.42 In the dehydrohalogenation of...Ch. 8 - Prob. 8.43PCh. 8 - Prob. 8.44PCh. 8 - Prob. 8.45PCh. 8 - Prob. 8.46PCh. 8 - Prob. 8.47PCh. 8 - Prob. 8.48PCh. 8 - What alkyl chloride affords the following alkene...Ch. 8 - Draw the products formed when each dihalide is...Ch. 8 - Draw the structure of a dihalide that could be...Ch. 8 - Under certain reaction conditions, 2,...Ch. 8 - For which reaction mechanisms, SN1, SN2, E1 or...Ch. 8 - Draw the organic products formed in each...Ch. 8 - Prob. 8.55PCh. 8 - Draw all products, including stereoisomers, in...Ch. 8 - Draw all of the substitution and elimination...Ch. 8 - Prob. 8.58PCh. 8 - 8.59 Draw a stepwise, detailed mechanism for each...Ch. 8 - Draw the major product formed when...Ch. 8 - Draw a stepwise, detailed mechanism for the...Ch. 8 - Explain why the reaction of with gives ...Ch. 8 - Draw a stepwise detailed mechanism that...Ch. 8 - Prob. 8.64PCh. 8 - 8.65 Explain the selectivity observed in the...Ch. 8 - Prob. 8.66PCh. 8 - Prob. 8.67PCh. 8 - 8.68 (a) Draw all products formed by treatment of...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Choose the best answer to each of the following. Explain your reasoning. If Earth were twice as far as it actua...
Cosmic Perspective Fundamentals
What process causes the Mediterranean intermediate Water MIW to become more dense than water in the adjacent At...
Applications and Investigations in Earth Science (9th Edition)
Give the IUPAC name for each compound.
Organic Chemistry
Why do scientists think that all forms of life on earth have a common origin?
Genetics: From Genes to Genomes
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
How could you separate a mixture of the following compounds? The reagents available to you are water, either, 1...
Organic Chemistry (8th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Calibri 11 + BIL NAME: Jaylena M A student is investigating the ctect of volume on pressure during a lab activity. The student uses the following volumes (mL). 12, 13, 14, 16, 17, 18, 19, 20, 21, 22, 23, 24, 26, 28, 30, 33, 34, 35, 38, 40, 42, 44. 46, and 50. As the volume changed they measured the following pressures (atm) 11.0, 10.5, 10.0, 9.2. 8.5, 78, 75, 7.0, 6.8, 6.5, 6.0, 5.9, 5.5, 5.0, 4.8, 4.5, 4.2, 3.9, 3.8, 3.5, 3.3, 3.2, 3.0, 2.9. What is the independent variable? Volume Imla What is the dependent variable? Pressure Jatm Use the data and make a PROPER data table. Volume 1mL) Pressure latm 110arrow_forwardDraw all resonance forms of the molecules. Include curved arrow notation. Label major resonance contributor.arrow_forward: Resonance Forms a) Draw all resonance forms of the molecules. Include curved arrow notation. Label major resonance contributor. SO₂ NO3arrow_forward
- 1d. Use Le Chatelier's principle to describe the effect of the following changes on the position of the Haber-Bosch equilibrium: N2(g) + 3H2(g)= 2NH3(9) AH = -92kJ Choose one of the following answers: shift to reactant side, shift to product side or no change and draw the resulting graph. I. Increase the [N2(g)] Effect: H₂ N₂ NH3 II. Decrease the volume of the container. Effect: H₂ N₂2 NH3arrow_forwardf) The unusual molecule [2.2.2] propellane is pictured. 1) Given the bond length and bond angles in the image, what hybridization scheme best describes the carbons marked by the askerisks? 2) What types of orbitals are used in the bond between the two carbons marked by the askerisks? 3) How does this bond compare to an ordinary carbon-carbon bond (which is usually 1.54 Å long)? CH2 1.60Å H₂C * H₂C CH2 C H2C * C Of H₂ 120°arrow_forwarde) Determine the hybridization and geometry around the indicated carbon atoms. H3C CH3 B HC CH2 A C C C CH3arrow_forward
- 75.0 grams of an unknown metal was heated to 95.0°C, it was then placed into 150.0 grams of water at23.1°C, when the metal and water reached thermal equilibrium, the temperature was 27.8°C. Calculatethe specific heat of the metal. (Assume that the specific heat of water is 4.18 J/g °C)arrow_forwardPlease correct answer and don't used hand raitingarrow_forwardA 25.0 g sample of water was cooled from 23.9°C to 12.7°C, how much heat was released? (Assume thatthe specific heat of water is 4.18 J/g °C)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License