
Concept explainers
Draw the organic products formed in each reaction.
a. 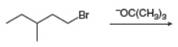 d.
d. 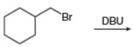 g.
g. 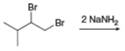
b. 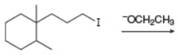 e.
e. 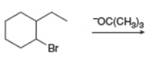 h.
h. 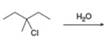
c. 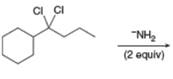 f.
f. 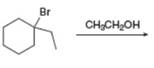
(a)
Interpretation: The organic product formed in the given reaction is to be drawn.
Concept introduction: The dehydrohalogenation reaction of primary alkyl halide mostly favors
Answer to Problem 8.54P
The organic product formed in the given reaction is,
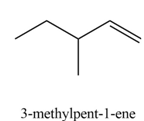
Explanation of Solution
The given reaction involves primary alkyl halide and strong bulky base. Primary alkyl halides can undergo substitution as well as elimination reactions. Since, the base is strong and bulky, it undergoes elimination reaction through
The corresponding reaction is shown below.
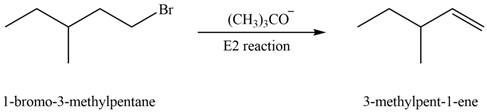
Figure 1
(a) The organic product formed in the given reaction is drawn in Figure 1.
(b)
Interpretation: The organic product formed in the given reaction is to be drawn.
Concept introduction: The dehydrohalogenation reaction of primary alkyl halide mostly favors
Answer to Problem 8.54P
The organic product formed in the given reaction is,
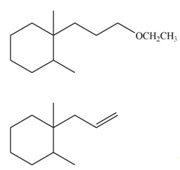
Explanation of Solution
In the given reaction, the alkyl halide is primary and base is strong, negatively charged base as well as nucleophile. Primary alkyl halides can undergo substitution as well as elimination reactions. Since, the base is strong, and also a strongnucleophile, the given halide undergoes substitution reaction through
The corresponding reaction is shown below.
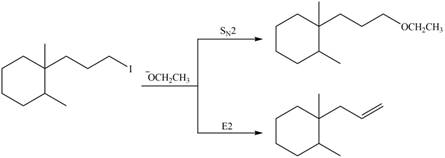
Figure 2
The organic product formed in the given reaction is drawn in Figure 2.
(c)
Interpretation: The organic product formed in the given reaction is to be drawn.
Concept introduction: A carbon atom bonded to two halogen groups is called germinal dihalides. Geminal dihalides undergo double dehydrohalogenation reaction in the presence of excess base to form alkyne as the final product. The reaction prefers elimination pathway.
Answer to Problem 8.54P
The organic product formed in the given reaction is,
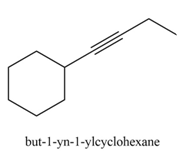
Explanation of Solution
In the given reaction, germinal dihalide is the starting material and amine base is strong, negatively chargedand present in excess amount
The corresponding reaction is shown below.
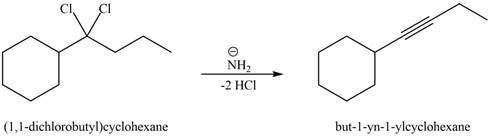
Figure 3
The organic product formed in the given reaction is drawn in Figure 3.
(d)
Interpretation: The organic product formed in the given reaction is to be drawn.
Concept introduction: The dehydrohalogenation reaction of primary alkyl halide mostly favors
Answer to Problem 8.54P
The organic product formed in the given reaction is,
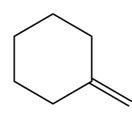
Explanation of Solution
In the given reaction, the alkyl halide is primary and base is strong, but non-nucleophilic. Primary alkyl halides can undergo substitution as well as elimination reactions. Since, the base is strong and non-nucleophilic, it undergoes elimination reaction through E2 pathway.
The corresponding reaction is shown below.
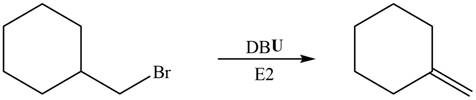
Figure 4
The organic product formed in the given reaction is drawn in Figure 4.
(e)
Interpretation: The organic product formed in the given reaction is to be drawn.
Concept introduction: The dehydrohalogenation reaction of primary alkyl halide mostly favors
Answer to Problem 8.54P
The organic product formed in the given reaction is,
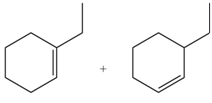
Explanation of Solution
In the given reaction, the alkyl halide is secondary and base is a strong, negatively charged bulky base. Secondaryalkyl halides can undergo substitution as well as elimination reactions. Since, the base is strong and bulky it undergoes elimination reaction through E2 pathway.
The corresponding reaction is shown below.
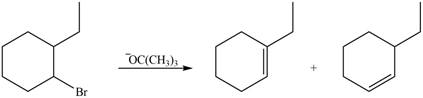
Figure 5
The organic product formed in the given reaction is drawn in Figure 5.
(f)
Interpretation: The organic product formed in the given reaction is to be drawn.
Concept introduction: The dehydrohalogenation reaction of primary alkyl halide mostly favors
Answer to Problem 8.54P
The organic product formed in the given reaction is,
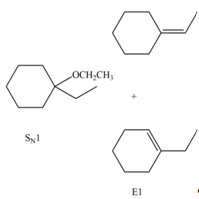
Explanation of Solution
The given reaction involves tertiary alkyl halide, weak base that is also a weak nucleophile. These conditions make the given halide (tertiary) suitable to undergo either substitution by
The corresponding reaction is shown below.
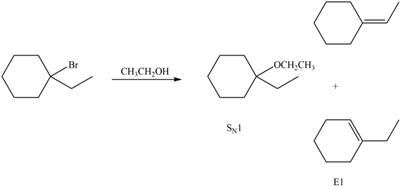
Figure 6
The organic product formed in the given reaction is drawn in Figure 6.
(g)
Interpretation: The organic product formed in the given reaction is to be drawn.
Concept introduction: Vicinal dihalides are the compounds in which the adjacent carbon atoms possess halogen groups (one on each carbon). These dihalides yield alkynes when treated with two equivalents of sodamide
Answer to Problem 8.54P
The organic product formed in the given reaction is,
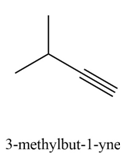
Explanation of Solution
The given alkyl halide is vicinal dihalide.
Vicinal dihalides are the compounds in which the adjacent carbon atoms possess halogen groups (one on each carbon). These dihalides yield alkynes when treated with two equivalents of sodamide
The corresponding reaction is shown below.
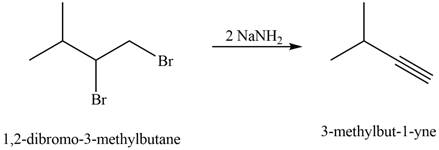
Figure 7
The organic product formed in the given reaction is drawn in Figure 7.
(h)
Interpretation: The organic product formed in the given reaction is to be drawn.
Concept introduction: The dehydrohalogenation reaction of alkyl halide mostly favors
Answer to Problem 8.54P
The organic product formed in the given reaction is,
Explanation of Solution
The given alkyl halide is tertiary. Water is a weak base. These conditions make the given halide (tertiary) suitable to undergo substitution as well as elimination reaction
The corresponding reaction is shown below.
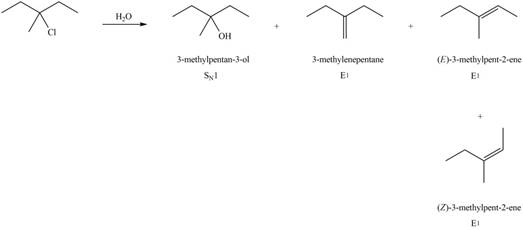
Figure 8
The organic product formed in the given reaction is drawn in Figure 8
Want to see more full solutions like this?
Chapter 8 Solutions
Package: Loose Leaf for Organic Chemistry with Biological Topics with Connect Access Card
Additional Science Textbook Solutions
Microbiology Fundamentals: A Clinical Approach
SEELEY'S ANATOMY+PHYSIOLOGY
Organic Chemistry
General, Organic, and Biological Chemistry - 4th edition
Physical Universe
Human Anatomy & Physiology (2nd Edition)
- 8. (16 pts) Provide the stepwise mechanism for the synthesis of the following compound via an enaminearrow_forwardDraw the titration curve of (i) weak acid vs. strong base; (ii) weak acid vs. weakbase; (iii) diprotic acid with strong base (iii) triprotic acid with strong base.arrow_forwardComplete the reaction in the drawing area below by adding the major products to the right-hand side. If there won't be any products, because nothing will happen under these reaction conditions, check the box under the drawing area instead. Note: if the products contain one or more pairs of enantiomers, don't worry about drawing each enantiomer with dash and wedge bonds. Just draw one molecule to represent each pair of enantiomers, using line bonds at the chiral center. More... No reaction. my ㄖˋ + 1. Na O Me Click and drag to start drawing a structure. 2. H +arrow_forward
- Predict the intermediate 1 and final product 2 of this organic reaction: NaOMe H+ + 1 2 H H work up You can draw 1 and 2 in any arrangement you like. Note: if either 1 or 2 consists of a pair of enantiomers, just draw one structure using line bonds instead of 3D (dash and wedge) bonds at the chiral center. Click and drag to start drawing a structure. X $ dmarrow_forwardPredict the major products of this organic reaction: 1. NaH (20°C) 2. CH3Br ? Some notes: • Draw only the major product, or products. You can draw them in any arrangement you like. • Be sure to use wedge and dash bonds where necessary, for example to distinguish between major products that are enantiomers. • If there are no products, just check the box under the drawing area. No reaction. Click and drag to start drawing a structure. G Crarrow_forwardPredict the major products of this organic reaction: 1. LDA (-78°C) ? 2. Br Some notes: • Draw only the major product, or products. You can draw them in any arrangement you like. . • Be sure to use wedge and dash bonds where necessary, for example to distinguish between major products that are enantiomers. • If there are no products, just check the box under the drawing area. No reaction. Click and drag to start drawing a structure. Xarrow_forward
- Please draw the structuresarrow_forwardDraw the missing intermediates 1 and 2, plus the final product 3, of this synthesis: 0 1. Eto 1. Eto- 1 2 2. MeBr 2. EtBr H3O+ A 3 You can draw the three structures in any arrangement you like. Explanation Check Click and drag to start drawing a structure.arrow_forwardDraw the missing intermediate 1 and final product 2 of this synthesis: 1. MeO- H3O+ 1 2 2. PrBr Δ You can draw the two structures in any arrangement you like. Click and drag to start drawing a structure.arrow_forward
- What is the differences between: Glyceride and phosphoglyceride Wax and Fat Soap and Fatty acid HDL and LDL cholesterol Phospho lipids and sphingosine What are the types of lipids? What are the main lipid components of membrane structures? How could lipids play important rules as signaling molecules and building units? The structure variety of lipids makes them to play significant rules in our body, conclude breifly on this statement.arrow_forwardWhat is the differences between DNA and RNA for the following: - structure - function - type What is the meaning of: - replication - transcription - translation show the base pair connection(hydrogen bond) in DNA and RNAarrow_forwardWhat is the IP for a amino acid- give an example what are the types of amino acids What are the structures of proteins The N-Terminal analysis by the Edman method shows saralasin contains sarcosine at the N-terminus. Partial hydrolysis of saralasin with dilute hydrochloric acid yields the following fragments: Try-Val-His Sar-Arg-Val His-Pro-Ala Val- Tyr- Val Arg-Val-Tyr What is the structure of saralasin?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





