
Concept explainers
(a) Draw all products formed by treatment of each dibromide (A and B) with one equivalent of
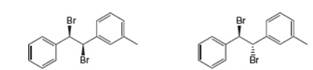
A B
(a)
Interpretation: All products formed by the treatment of the given dibromide (A and B) with one equivalent of
Concept introduction: The two-step unimolecular elimination reaction that favors the removal of a HX substituent and the formation of a carbocation intermediate takes place in its first step. In the second step of the reaction, the carbocation forms a double bond. This type of reaction is termed E1 elimination reaction.
The one-step bimolecular elimination reaction that favors the removal of a proton by a base from carbon adjacent to the leaving group that results in the formation of a carbocation is termed as
Answer to Problem 8.68P
The products that formed by the treatment of the given dibromide A with one equivalent of
Explanation of Solution
The reaction of dibromide A with one equivalent of
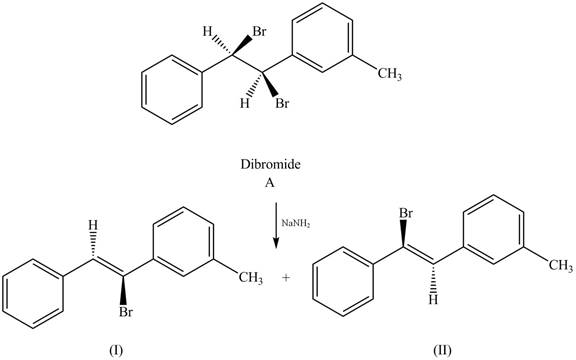
Figure 1
In this reaction, the given dihalide undergoes elimination reaction in the presence of one equivalent of the strong base,
The reaction of dibromide B with one equivalent of
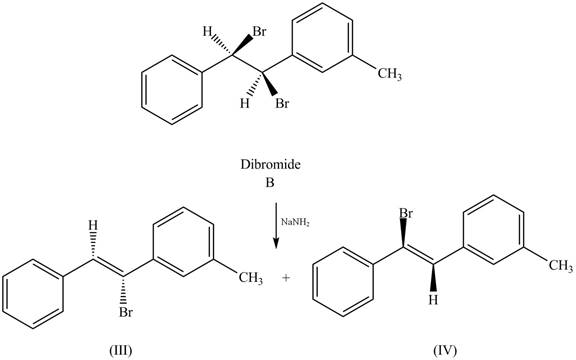
Figure 2
In this reaction, the given dihalide undergoes elimination reaction in the presence of one equivalent of the strong base,
The products that formed by the treatment of the given dibromide A with one equivalent of
(b)
Interpretation: The labeling of the pairs of diastereomers and constitutional isomers are to be shown.
Concept introduction: Diastereomers compounds are not mirror images of each other and are different in configuration at one or more stereocentres.
The isomers which have same molecular formula but different connectivity of atoms are constitutional isomers.
Answer to Problem 8.68P
The labeling of the pairs of diastereomers is,
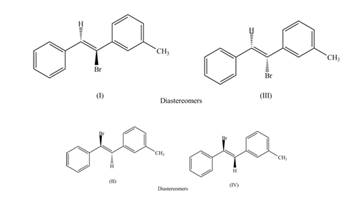
The labeling of the pairs of constitutional isomers is,
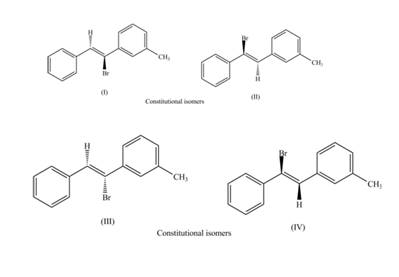
Explanation of Solution
The first pair of diastereomers is shown as,
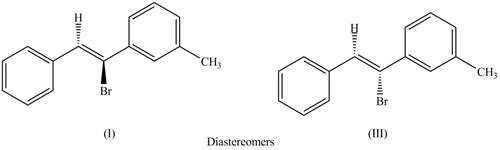
Figure 3
Thus, the products (I) and (III) are diastereomers that are not mirror images of each other and are differ in configuration.
The second pair of diastereomers is shown as,
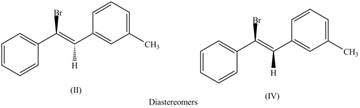
Figure 4
Thus, the products (II) and (IV) are diastereomers that are not mirror images of each other and are differ in configuration. (I), (II) and (III),(IV) are constitutional isomers as they differ only in arrangement of bonds.
The first pair of constitutional isomers is shown as,
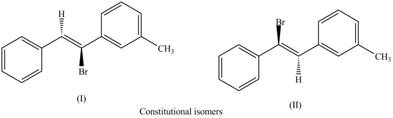
Figure 5
Thus, the products (I) and (II) are constitutional isomers as they possess different arrangement of bonds.
The first pair of constitutional isomers is shown as,
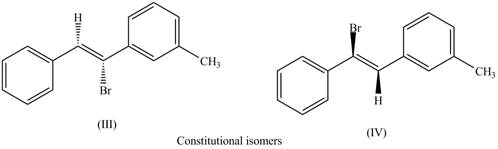
Figure 6
Thus, the products (III) and (IV) are constitutional isomers as they possess different arrangement of bonds.
The labeling of the pairs of diastereomers is shown in Figure 3 and Figure 4. The labeling of the pairs of constitutional isomers is shown in Figure 5 and Figure 6.
Want to see more full solutions like this?
Chapter 8 Solutions
Package: Loose Leaf for Organic Chemistry with Biological Topics with Connect Access Card
Additional Science Textbook Solutions
Organic Chemistry (8th Edition)
MARINE BIOLOGY
Biology: Life on Earth (11th Edition)
HUMAN ANATOMY
Human Physiology: An Integrated Approach (8th Edition)
- Rank the following compounds in order of decreasing dipole moment. |>||>||| ||>|||>| |>|||>|| |||>||>| O ||>>||| H F H F H c=c || H c=c F F IIIarrow_forwardchoose the description that best describes the geometry for the following charged species ch3-arrow_forwardWhy isn't the ketone in this compound converted to an acetal or hemiacetal by the alcohol and acid?arrow_forward
- What is the approximate bond angle around the nitrogen atom? HNH H Harrow_forwardOH 1. NaOCH2CH3 Q 2. CH3CH2Br (1 equiv) H3O+ Select to Draw 1. NaOCH2 CH3 2. CH3Br (1 equiv) heat Select to Edit Select to Drawarrow_forwardComplete and balance the following half-reaction in acidic solution. Be sure to include the proper phases for all species within the reaction. S₂O₃²⁻(aq) → S₄O₆²⁻(aq)arrow_forward
- Q Select to Edit NH3 (CH3)2CHCI (1 equiv) AICI 3 Select to Draw cat. H2SO4 SO3 (1 equiv) HO SOCl2 pyridine Select to Edit >arrow_forwardComplete and balance the following half-reaction in basic solution. Be sure to include the proper phases for all species within the reaction. Zn(s) → Zn(OH)₄²⁻(aq)arrow_forwardb. ὋΗ CH3CH2OH H2SO4arrow_forward
- For the reaction A (g) → 3 B (g), Kp = 0.379 at 298 K. What is the value of ∆G for this reaction at 298 K when the partial pressures of A and B are 5.70 atm and 0.250 atm?arrow_forward14. Calculate the concentrations of Ag+, Ag(S2O3), and Ag(S2O3)23- in a solution prepared by mixing 150.0 mL of 1.00×10-3 M AgNO3 with 200.0 mL of 5.00 M Na2S2O3 Ag+ + S20 Ag(S203)¯ K₁ = 7.4 × 108 Ag(S203)¯ + S20¯ = Ag(S203) K₂ = 3.9 x 104arrow_forwardΗΝ, cyclohexanone pH 4-5 Draw Enamine I I CH3CH2Br THF, reflux H3O+ I Drawing Draw Iminium Ionarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





