
(a)
Interpretation:
The number of electrons in an atom that can have
Concept introduction:
The quantum numbers provide complete information about the electron. There are four quantum numbers as follows:
1. The principal quantum number and it is represented by n. It tells about the shell to which the electron belongs.
2. The azimuthal quantum number and it is represented by l. It tells about the subshell of the electrons.
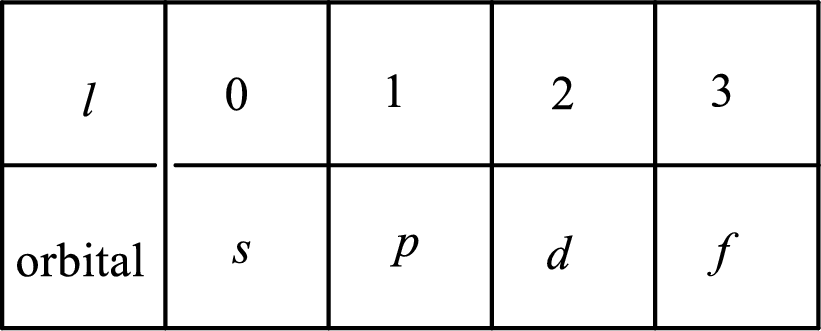
3. The magnetic quantum number and it is represented by
4. The spin quantum number and it is represented by
(b)
Interpretation:
The number of electrons that an atom with sublevel designation
Concept introduction:
The quantum numbers provide complete information about the electron. There are four quantum numbers as follows:
1. The principal quantum number and it is represented by n. It tells about the shell to which the electron belongs.
2. The azimuthal quantum number and it is represented by l. It tells about the subshell of the electrons.
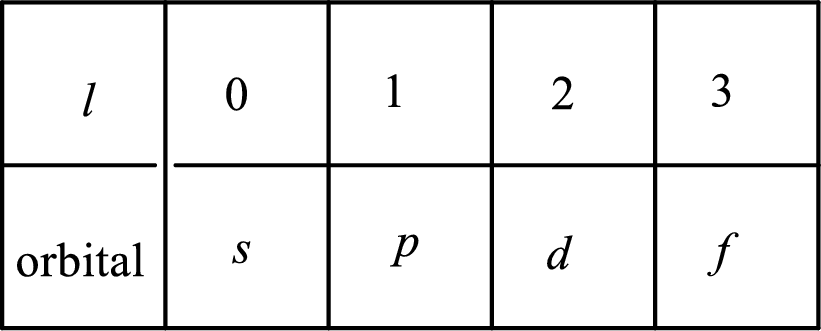
3. The magnetic quantum number and it is represented by
4. The spin quantum number and it is represented by
(c)
Interpretation:
The number of electrons that an atom with sublevel designation
Concept introduction:
The quantum numbers provide complete information about the electron. There are four quantum numbers as follows:
1. The principal quantum number and it is represented by n. It tells about the shell to which the electron belongs.
2. The azimuthal quantum number and it is represented by l. It tells about the subshell of the electrons.
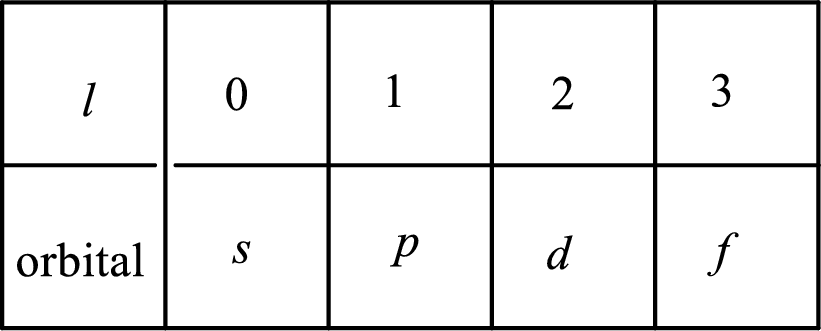
3. The magnetic quantum number and it is represented by
4. The spin quantum number and it is represented by
Want to see the full answer?
Check out a sample textbook solution
Chapter 8 Solutions
Loose Leaf for Chemistry: The Molecular Nature of Matter and Change
- Nonearrow_forwardThe U. S. Environmental Protection Agency (EPA) sets limits on healthful levels of air pollutants. The maximum level that the EPA considers safe for lead air pollution is 1.5 μg/m3 . If your lungs were filled with air containing this level of lead, how many lead atoms would be in your lungs? (Assume a total lung volume of 5.40 Larrow_forwardDuring a(n) ________ process, energy is transferred from the system to the surroundings. exothermic endothermic thermodynamic thermochemical physicalarrow_forward
- Use the following information to determine the enthalpy for the reaction shown below. → S(s) + O2(g) SO2(9) ΔΗ Π ? Reference reactions: S(s) + O2(g) SO3(9) 2SO2(g) + O2(g) → 2SO3(g) AHxn = -395kJ AHrxn = ― -198kJarrow_forwardQ1. (a) Draw equations for homolytic and heterolytic cleavages of the N-H bond in NH3. Use curved arrows to show the electron movement. (b) Draw equations for homolytic and heterolytic cleavages of the N-H bond in NH4*. Use curved arrows to show the electron movement.arrow_forwardIndicate which of the following is not an element in its standard state at 25oC and 1 atm. Group of answer choices O2(g) H2(g) Ne(g) N(g) C(s, graphite)arrow_forward
- 6. Show how you would accomplish the following transformations. (Show the steps and reagents/solvents needed) 2-methylpropene →2,2-dimethyloxiran Iarrow_forward4) Answer the following exercise with curved arrows indicating who is a nucleophile or Who is the electrophile? 2.44 Predict the structure of the product formed in the reaction of the organic base pyridine with the organic acid acetic acid, and use curved arrows to indicate the direction of electron flow. 7 H3C OH N Pyridine Acetic acidarrow_forwardUsing the data provided please help me answer this question. Determine the concentration of the iron(Ill) salicylate in the unknown directly from to graph and from the best fit trend-line (least squares analysis) of the graph that yielded a straight line.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





