
Concept explainers
8-112 Consider an initial 0.040 M hypobromous acid (HOBr) solution at a certain temperature.
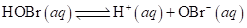
At equilibrium after partial dissociation, its pH is found to be 5.05. What is the acid ionization constant, Ka, for hypobromous acid at this temperature?
Interpretation:
The acid dissociation constant of hypobromous acid is to be calculated.
Concept Introduction:
Weak acids do not dissociate completely. Let HA be a weak acid. The dissociation of the weak acid can be represented by the chemical equation,
The equation for acid dissociation constant can be written from this chemical equation.
Here,
Answer to Problem 8.112P
The acid dissociation constant of hypobromous acid is
Explanation of Solution
Hypobromous acid is a weak acid. Hence, it do not dissociate completely. The dissociation of the given weak acid can be represented by the chemical equation,
The equation for acid dissociation constant can be written from this chemical equation.
The concentrations of each of the ions at equilibrium can be obtained from the ICE table. Where ICE represents the Initial, Change and Equilibrium concentrations of the weak acid.
The hydrogen ion concentration can be obtained from the given pH. The pH is defined as the negative logarithm of the hydrogen ion concentration.
The pH of the weak acid solution at equilibrium is 5.05. Thus, we can calculate the concentration of the hydrogen ion.
We calculated the “x” which is the concentration of hydrogen ion. The concentration of the anion is also “x”. Thus,
Now, we need to calculate the concentration of
The concentrations of the anion, hydrogen ion and hypobromous acid are used in the equation used for acid dissociation constant.
Thus, the acid dissociation constant of hypobromous acid is
Weak acids do not dissociate completely. Each weak acid has a specific dissociation constant. Here, ICE table is made from the given chemical equation. Thus, the acid dissociation constant of hypobromous acid is
Want to see more full solutions like this?
Chapter 8 Solutions
Introduction to General, Organic and Biochemistry
- Q10: (a) Propose a synthesis of C from A. (b) Propose a synthesis of C from B. Br Br ...\SCH 3 A B Carrow_forward9: Complete the missing entities for following reactions (e.g., major product(s), reactants, and/or solvents) for the SN2 reactions to occur efficiently. Include curved-arrow mechanism for reactions a) to d).arrow_forwardComplete the missing entities for following reactions (e.g., major product(s), reactants, and/or solvents) for the SN2 reactions to occur efficiently. Include curved-arrow mechanism for reactions a) to d).arrow_forward
- QUESTION 3: Provide the synthetic steps that convert the starting material into the product (no mechanism required). HO OH NH CH3 multiple steps 요요 H3Carrow_forwardQ6: Predict the effect of the changes given on the rate of the reaction below. CH3OH CH3Cl + NaOCH3 → CH3OCH3 + NaCl a) Change the substrate from CH3CI to CH31: b) Change the nucleophile from NaOCH 3 to NaSCH3: c) Change the substrate from CH3CI to (CH3)2CHCI: d) Change the solvent from CH3OH to DMSO.arrow_forwardQ3: Arrange each group of compounds from fastest SN2 reaction rate to slowest SN2 reaction rate. a) CI Cl فيكم H3C-Cl A B C D Br Br b) A B C Br H3C-Br Darrow_forward
- Q2: Group these solvents into either protic solvents or aprotic solvents. Acetonitrile (CH3CN), H₂O, Acetic acid (CH3COOH), Acetone (CH3COCH3), CH3CH2OH, DMSO (CH3SOCH3), DMF (HCON(CH3)2), CH3OHarrow_forwardSuppose the rate of evaporation in a hot, dry region is 1.76 meters per year, and the seawater there has a salinity of 35 ‰. Assuming a 93% yield, how much salt (NaCl) can be harvested each year from 1 km2 of solar evaporation ponds that use this seawater as a source?arrow_forwardhelparrow_forward
- Explain why only the lone pairs on the central atom are taken into consideration when predicting molecular shapearrow_forward(ME EX1) Prblm #9/10 Can you explain in detail (step by step) I'm so confused with these problems. For turmber 13 can u turn them into lewis dot structures so I can better understand because, and then as well explain the resonance structure part. Thanks for the help.arrow_forwardProblems 19 and 20: (ME EX1) Can you please explain the following in detail? I'm having trouble understanding them. Both problems are difficult for me to explain in detail, so please include the drawings and answers.arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning





