
CONNECT IA GENERAL ORGANIC&BIO CHEMISTRY
4th Edition
ISBN: 9781260562620
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 8, Problem 78P
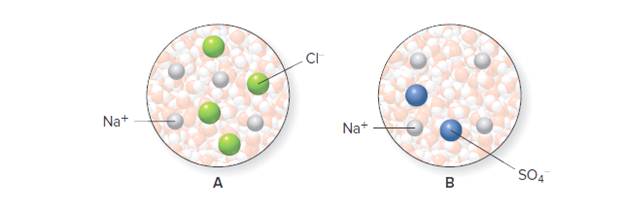
Representations A (containing 1.0 mol ofNaCl) and B (containing 0.5 mol of
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Predict the major organic product(s), if any, of the following reactions. Assume all reagents are in excess unless otherwise indicated.
Predict the major organic product(s), if any, of the following reactions. Assume all reagents are in excess unless otherwise indicated.
How many signals would you expect to find in the 1 H NMR spectrum of each given compound?
Part 1 of 2
2
Part 2 of 2
HO
5
☑
Х
IIIIII*****
§
Chapter 8 Solutions
CONNECT IA GENERAL ORGANIC&BIO CHEMISTRY
Ch. 8.1 - Classify each substance as a heterogeneous...Ch. 8.1 - Use the appearance of each product to classify it...Ch. 8.2 - Consider the following diagrams for an aqueous...Ch. 8.2 - Classify each solution as an electrolyte or...Ch. 8.2 - Using the given number of moles, determine how...Ch. 8.2 - Prob. 8.2PPCh. 8.2 - A solution contains the following ions:...Ch. 8.2 - If a solution contains 125 mEq of Na+ per liter,...Ch. 8.3 - Which compounds are water soluble? a. NaNO3 b. CH4...Ch. 8.3 - Prob. 8.7P
Ch. 8.3 - Use the solubility rules to predict whether the...Ch. 8.3 - Use the solubility rules for ionic compounds to...Ch. 8.4 - Why does a soft drink become "flat" faster when it...Ch. 8.4 - Predict the effect each change has on the...Ch. 8.5 - A commercial mouthwash contains 4.3 g of ethanol...Ch. 8.5 - What is the weight/volume percent concentration of...Ch. 8.5 - Prob. 8.6PPCh. 8.5 - Prob. 8.7PPCh. 8.5 - A drink sold in a health food store contains 0.50%...Ch. 8.5 - Prob. 8.12PCh. 8.5 - What is the concentration in parts per million of...Ch. 8.6 - Prob. 8.10PPCh. 8.6 - Prob. 8.13PCh. 8.6 - Prob. 8.11PPCh. 8.6 - Prob. 8.12PPCh. 8.6 - How many grams of NaCl are contained in each of...Ch. 8.6 - How many milliliters of a 0.25 M sucrose solution...Ch. 8.7 - What is the concentration of a solution formed by...Ch. 8.7 - If the solution of A+B- in X is diluted, which...Ch. 8.7 - Prob. 8.15PPCh. 8.7 - Prob. 8.16PCh. 8.8 - What is the boiling point of a solution prepared...Ch. 8.8 - Representations A, B, and C each show an aqueous...Ch. 8.8 - Prob. 8.18PPCh. 8.8 - What is the melting point of a solution that is...Ch. 8.9 - Which solution in each pair exerts the greater...Ch. 8.9 - Prob. 8.19PCh. 8.9 - Consider the two aqueous solutions separated by a...Ch. 8.9 - What happens to a red blood cell when it is placed...Ch. 8 - Prob. 21PCh. 8 - Prob. 22PCh. 8 - Prob. 23PCh. 8 - Which representation of molecular art better shows...Ch. 8 - Classify each of the following as a solution,...Ch. 8 - Classify each of the following as a solution,...Ch. 8 - Prob. 27PCh. 8 - Label each diagram as a strong electrolyte, weak...Ch. 8 - Prob. 29PCh. 8 - Prob. 30PCh. 8 - Prob. 31PCh. 8 - Prob. 32PCh. 8 - Consider a mixture of two substances shown in blue...Ch. 8 - Which diagram (C or D) best represents what occurs...Ch. 8 - If the solubilityofKClin 100 mL of H2O is 34 g at...Ch. 8 - If the solubilityofsucrosein 100 mL of H2O is 204...Ch. 8 - Prob. 37PCh. 8 - Prob. 38PCh. 8 - Using the ball-and-stick model for methanol...Ch. 8 - Prob. 40PCh. 8 - Prob. 41PCh. 8 - Prob. 42PCh. 8 - Prob. 43PCh. 8 - Prob. 44PCh. 8 - Prob. 45PCh. 8 - Prob. 46PCh. 8 - Prob. 47PCh. 8 - How is the solubility of helium gas in water...Ch. 8 - Use the solubility rules listed in Section 8.3B to...Ch. 8 - Use the solubility rules listed in Section 8.3B to...Ch. 8 - Prob. 51PCh. 8 - Prob. 52PCh. 8 - Prob. 53PCh. 8 - Prob. 54PCh. 8 - Prob. 55PCh. 8 - Prob. 56PCh. 8 - Prob. 57PCh. 8 - Prob. 58PCh. 8 - How would you use a 250-mL volumetric flask to...Ch. 8 - How would you use a 250-mLvolumetric flask to...Ch. 8 - Prob. 61PCh. 8 - Prob. 62PCh. 8 - Prob. 63PCh. 8 - Prob. 64PCh. 8 - Prob. 65PCh. 8 - What is the molarity of a 20.0% (v/v) aqueous...Ch. 8 - Prob. 67PCh. 8 - Prob. 68PCh. 8 - Prob. 69PCh. 8 - Prob. 70PCh. 8 - Prob. 71PCh. 8 - Prob. 72PCh. 8 - Prob. 73PCh. 8 - Prob. 74PCh. 8 - Prob. 75PCh. 8 - Prob. 76PCh. 8 - Prob. 77PCh. 8 - Representations A (containing 1.0 mol ofNaCl) and...Ch. 8 - What is the boiling point of a solution that...Ch. 8 - Prob. 80PCh. 8 - If 150 g of ethylene glycol (C2H6O2) is added to...Ch. 8 - Prob. 82PCh. 8 - Prob. 83PCh. 8 - Prob. 84PCh. 8 - Which solution in each pair has the higher melting...Ch. 8 - Prob. 86PCh. 8 - A flask contains two compartments (A and B) with...Ch. 8 - A flask contains two compartments (A and B) with...Ch. 8 - The molecular art illustrates a red blood cell in...Ch. 8 - Prob. 90PCh. 8 - Prob. 91PCh. 8 - Explain why more sugar dissolves in a cup of hot...Ch. 8 - If the concentration of glucose in the blood is...Ch. 8 - Prob. 94PCh. 8 - Mannitol, a carbohydrate, is supplied as a 25%...Ch. 8 - A patient receives 750 ml, of a 10.% (w/v) aqueous...Ch. 8 - Explain why a cucumber placed in a concentrated...Ch. 8 - Explain why a cucumber placed in a concentrated...Ch. 8 - Prob. 99PCh. 8 - Prob. 100PCh. 8 - Prob. 101PCh. 8 - Prob. 102PCh. 8 - The therapeutic concentration—the concentration...Ch. 8 - Prob. 104CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A carbonyl compound has a molecular ion with a m/z of 86. The mass spectra of this compound also has a base peak with a m/z of 57. Draw the correct structure of this molecule. Drawingarrow_forwardCan you draw this using Lewis dot structures and full structures in the same way they are so that I can better visualize them and then determine resonance?arrow_forwardSynthesize the following compound from cyclohexanol, ethanol, and any other needed reagentsarrow_forward
- For a titration of 20.00 mL of 0.0500 M H2SO4 with 0.100 M KOH, calculate the pH at each of the following volume of KOH used in the titration: 1) before the titration begin; 2) 10.00 mL; 3) 20.00 mL; 4) 30.00 mL. Ka2 = 1.20×10-2 for H2SO4.arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s) Be sure to account for all bond-breaking and bond-making steps Problem 73 of 10 Drawing Amows ro HO Donearrow_forward12. Synthesize the following target molecules (TMs) using the specified starting materials. .CI a) HO3S SM TM b) HO- SMarrow_forward
- For a titration of 20.00 mL of 0.0500 M H2SO4 with 0.100 M KOH, calculate the pH at each of the following volume of KOH used in the titration: 1) before the titration begin; 2) 10.00 mL; 3) 20.00 mL; 4) 30.00 mL. Ka2 = 1.20×10-2 for H2SO4.arrow_forwardWrite the systematic name of each organic molecule: structure name show work. don't give Ai generated solutionarrow_forwardShow work with explanation needed. Don't give Ai generated solutionarrow_forward
- A Elschboard Part of SpeechT-D Alt Leaming App app.aktiv.com Curved arrows are used to illustrate the flow of electrons. Using the provided resonance structures, draw the curved electron- pushing arrows to show the interconversion between resonance hybrid contributors. Be sure to account for all bond-breaking and bond-making steps. Include all lone pairs and formal charges in the structures. Problem 45 of 10 I Select to Add Arrows N Please selarrow_forwardSo I'm working on molecular geometry. Can you help me with this stuff here and create three circles: one that's 120, one that’s 180, and one that’s 109.5?arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. Problem 164 of N Select to Add Arrows CHI CH 1 1 1 Parrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning
Solutions: Crash Course Chemistry #27; Author: Crash Course;https://www.youtube.com/watch?v=9h2f1Bjr0p4;License: Standard YouTube License, CC-BY