
EP BASIC CHEMISTRY-STANDALONE ACCESS
6th Edition
ISBN: 9780134999890
Author: Timberlake
Publisher: PEARSON CO
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 8, Problem 37UTC
The chapter sections to review are shown in parentheses at the end of each problem.
If red spheres represent oxygen atoms, blue spheres represent nitrogen atoms, and all the molecules are gases, (8.1, 8.2, 8.3)
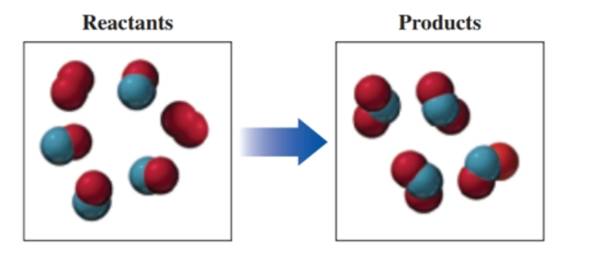
a. write the formula for each of the reactants and products.
b. write a balanced equation for the reaction.
c. indicate the type of reaction as combination, decomposition, single replacement, double replacement, or combustion.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Question 7.102
(5.8)Which of the following reactions will form a gaseous product?
O H₂CO3(aq) + Pb(NO3)2(aq)
O NaOH(aq) + HNO3(aq)
O None of these
O Na₂SO3(aq) + H₂SO4(aq)
◄ Previous
Need typed solution
Chapter 8 Solutions
EP BASIC CHEMISTRY-STANDALONE ACCESS
Ch. 8.1 - State the number of atoms of oxygen in the...Ch. 8.1 - State the number of atoms of oxygen in the...Ch. 8.1 - Prob. 3PPCh. 8.1 - Determine whether each of the following equations...Ch. 8.1 - All of the following are balanced equations. State...Ch. 8.1 - All of the following are balanced equations. State...Ch. 8.2 - Balance each of the following chemical...Ch. 8.2 - Balance each of the following chemical...Ch. 8.2 - Prob. 9PPCh. 8.2 - Prob. 10PP
Ch. 8.2 - Balance each of the following chemical...Ch. 8.2 - Prob. 12PPCh. 8.2 - Write a balanced equation using the correct...Ch. 8.2 - Write a balanced equation using the correct...Ch. 8.2 - Dinitrogen oxide, also known as laughing gas, is a...Ch. 8.2 - When ethanol C2H6O(aq) is consumed, it reacts with...Ch. 8.2 - In the body, the amino acid alanine C3H7NO2(aq)...Ch. 8.2 - Prob. 18PPCh. 8.3 - Classify each of the following as a combination,...Ch. 8.3 - Classify each of the following as a combination,...Ch. 8.3 - Classify each of the following as a combination,...Ch. 8.3 - Classify each of the following as a combination,...Ch. 8.3 - Using Table 8.3, predict the products that would...Ch. 8.3 - Using Table 8.3, predict the products that would...Ch. 8.4 - Prob. 25PPCh. 8.4 - Identify each of the following as an oxidation or...Ch. 8.4 - Prob. 27PPCh. 8.4 - In each of the following, identify the reactant...Ch. 8.4 - In the mitochondria of human cells, energy is...Ch. 8.4 - Prob. 30PPCh. 8.4 - When linoleic acid, an unsaturated fatty acid,...Ch. 8.4 - Prob. 32PPCh. 8.4 - a. During cellular respiration, aqueous glucose...Ch. 8.4 - Aqueous fatty acids undergo reaction with oxygen...Ch. 8 - The chapter sections to review are shown in...Ch. 8 - The chapter sections to review are shown in...Ch. 8 - The chapter sections to review are shown in...Ch. 8 - The chapter sections to review are shown in...Ch. 8 - Prob. 39UTCCh. 8 - The chapter sections to review are shown in...Ch. 8 - Prob. 41UTCCh. 8 - If blue spheres represent nitrogen atoms, purple...Ch. 8 - Identify the type of reaction for each of the...Ch. 8 - Identify the type of reaction for each of the...Ch. 8 - Balance each of the following chemical equations,...Ch. 8 - Balance each of the following chemical equations,...Ch. 8 - Predict the products and write a balanced equation...Ch. 8 - Prob. 48APPCh. 8 - Write a balanced equation for each of the...Ch. 8 - Write a balanced equation for each of the...Ch. 8 - Prob. 51APPCh. 8 - Prob. 52APPCh. 8 - Prob. 53CPCh. 8 - Prob. 54CPCh. 8 - Prob. 55CPCh. 8 - Prob. 56CPCh. 8 - The following problems are related to the topics...Ch. 8 - Prob. 58CPCh. 8 - Prob. 59CPCh. 8 - In the following diagram, if red spheres are the...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- One of the reactions in the industrial production ofnitric acid involves the production of nitric oxide: 4 NH3(g) 1 5 O2(g) → 4 NO(g) 1 6 H2O(g) (7.4) T/I a) If 4500 kg of ammonia, NH3(g), react with7500 kg of O2, what mass of NO will form? (b) What mass of the excess reagent will remain?arrow_forwardWrite the structures or molecular formulas of the possible reactants or productsWrite the structures or molecular formulas of the possible reactants or products.arrow_forward20 cm3 of hydrogen peroxide solution of unknown concentration reacts completely with 12.15 cm3 0.04 mol/dm3 of potassium permanganate solution in sulfuric acid media. Calculate the mass concentration of hydrogen peroxide in g/dm3. How many cm3 (USC) of oxygen is formed? (2.07; 29.77)arrow_forward
- Only 1 to 10arrow_forward(5.2, Similar to For More Practice 5.1) What mass of NaOH (in grams) do you need to make 250.0 mL of a 1.50 M NaOH solution? O 6.67 g O 22.3 g O 44.6 g O 15.0 garrow_forwardIn the experiment, we will perform a thermal decomposition reaction by heating a sample to cause the waters of hydration in the compound to leave.arrow_forward
- Nonearrow_forward5) How many moles of sulfuric acid are present in 0.500 L of 0.150 M solution? (0.0750) 6) How many grams of sulfuric acid are in the above solution? (7.35)arrow_forwardshould react most rapidly with Clz and AICI. (9.28 h) 8. Of the compounds shown below, NO2 HO. D. 9. Qarrow_forward
- 24. Calculate the mass percent of K in K2CrO4. (3.5)arrow_forwardComplete the following equations by writing the formula(s) of the product(s) and balancing the equation with coefficients. Use the type of reaction to aid in determining the products. 6. 7. 8. 9. Balanced Equation 10. Zn + FeCl3 + 12. C3H8 + HgO→ 13. Al + H₂SO4 → AgNO3 → 0₂ Cl₂ → Balanced Equation 11. Iron and oxygen react to form iron (III) oxide. afe +302 afe 203 Type of Reaction Single Replacement Write the balanced chemical equation for the following chemical reactions. Include the correct formulas for all reactants and products and balance with coefficients. Identify the type of reaction in the right column. Double Replacement Combustions Decomposition Combination Silver nitrate and magnesium chloride react to form magnesium nitrate and silver chloride. 2Aguo3 + MgCl₂ → 2 Agcl + ing [Nosta double displacement Type of Reaction Compustion Aluminum reacts with copper (II) sulfate to form aluminum sulfate and copper solid. 3 cu 50₂ +2A1-3 Cu +3504 +2A¹a (564)3 single displacment 10arrow_forward9. Which element is oxidized in the following reaction: 2FECI2 + Cl2 → 2FECI3 if Fe goes from +2 to +3 and CI goes from 0 to –2? Fe Neither element CI Cl and Fe GET IT NOW New version available! (3.0.220) PREVIOUSarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
NEET Chemistry | Group 14 Carbon Family | Theory & Problem Solving | In English | Misostudy; Author: Misostudy;https://www.youtube.com/watch?v=enOGIrcHh54;License: Standard YouTube License, CC-BY