
Concept explainers
Draw Lewis structures for the following organic molecules: (a) methanol
Interpretation:
Lewisstructure for the given organic molecules are to be drawn.
Concept introduction:
The octet rule states that every element in a main group must have eight electrons in its valence shell in order to attain noble gas configuration.
Valence electrons are the electrons that are present in the last(outer) shell of an element.
Lewis dot symbols contain dotsthat provides information about valence electrons.
Lewis structures depict bonds as lines and lone pairs as dots.
Answer to Problem 120AP
Solution:
(a)
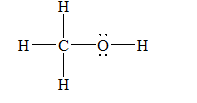
(b)
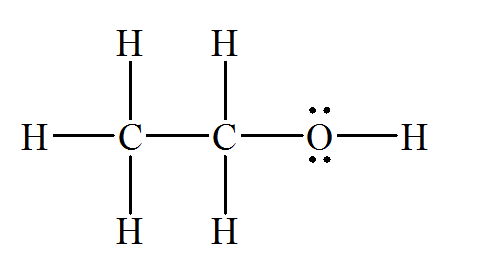
(c)
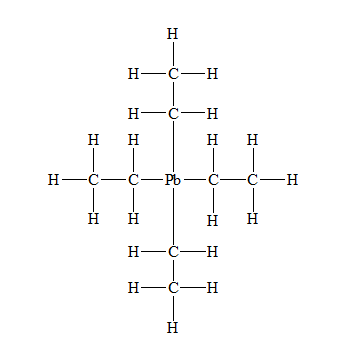
(d)
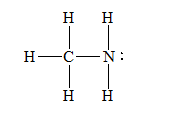
(e)
(f)
(g)
Explanation of Solution
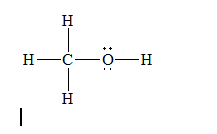
a)Methanol (
There are a total 14 electrons in
The skeletal structure for
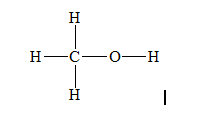
The Lewis structure for
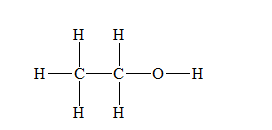
b) Ethanol (
There are a total 20 electrons in
The skeletal structure for
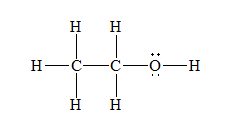
The Lewis structure for
c) Tetraethyl lead (
There are a total 56 electrons in
The Lewis structure for

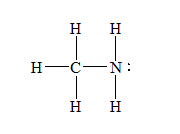
d) Methylamine,
There are a total 14 electrons in
The skeletal structure for
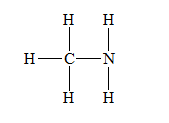
The Lewis structure for
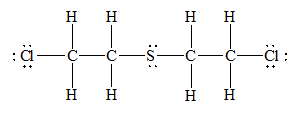
e) Mustard gas, (
There are a total 44 electrons in
The skeletal structure for
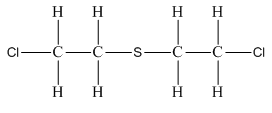
The Lewis structure for

Hence, the Lewis dot structure has beendrawn.
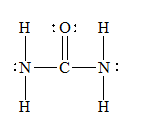
f) Urea (
There are a total 24 electrons in
The skeletal structure for
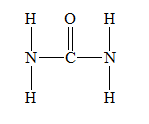
The Lewis structure for
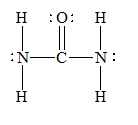
Given information:
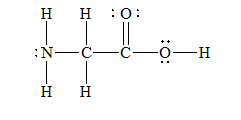
g) Glycine
There are a total 30 electrons in
The skeletal structure for
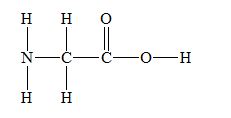
The Lewis structure for

Want to see more full solutions like this?
Chapter 8 Solutions
BURDGE CHEMISTRY VALUE ED (LL)
- Indicate whether the following two statements are correct or not:- The S8 heterocycle is the origin of a family of compounds- Most of the elements that give rise to stable heterocycles belong to group d.arrow_forwardcould someone draw curly arrow mechanism for this question pleasearrow_forwardIn the phase diagram of quartz (SiO2), indicate what happens as the pressure increases.arrow_forward
- Show work. Don't give Ai generated solutionarrow_forwardNonearrow_forwardTransmitance 3. Which one of the following compounds corresponds to this IR spectrum? Point out the absorption band(s) that helped you decide. OH H3C OH H₂C CH3 H3C CH3 H3C INFRARED SPECTRUM 0.8- 0.6 0.4- 0.2 3000 2000 1000 Wavenumber (cm-1) 4. Consider this compound: H3C On the structure above, label the different types of H's as A, B, C, etc. In table form, list the labeled signals, and for each one state the number of hydrogens, their shifts, and the splitting you would observe for these hydrogens in the ¹H NMR spectrum. Label # of hydrogens splitting Shift (2)arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning


