
Concept explainers
Write Lewis dot symbols for the following atoms and ions:
Interpretation:
The Lewis dot symbols for the given atoms and ions are to be written.
Concept introduction:
Lewis dot symbols contain dots that give information about the valence electrons.
Lewis dot symbols show both bond and lone pairs as dots.
Lewis structures show bonds as lines and lone pairs as dots.
Answer to Problem 5QP
Solution:
a)
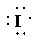
b)
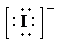
c)
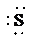
d)
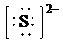
e)
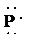
f)
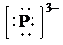
g)
h)
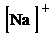
i)
j)
k)
l)
m)
n)
Explanation of Solution
a) Atom—
The number of valence electrons in
atom is
So, there are
So, the Lewis structure for
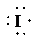
b) Ion—
The number of valence electrons in
So, there are
So, the Lewis structure for
is as follows:
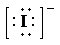
c) Atom—
The number of valence electrons in
atom is
So, there are 3 lone pairs present.
So, the Lewis structure for
is as follows:
d) Ion—
The number of valence electrons in
So, there are
So, the Lewis structure for
is as follows:
e) Atom—
The number of valence electrons in
atom is
So, there are
So, the Lewis structure for
is as follows:
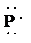
f) Ion—
The number of valence electrons in
So, there are
So, the Lewis structure for
is as follows:
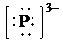
g) Atom—
The number of valence electrons in
atom is
So, there are no lone pairs present.
So, the Lewis structure for
is as follows:
h) Ion—
The number of valence electrons in
So, the Lewis structure for
is as follows:
i) Atom—
The number of valence electrons in
atom is
So, the Lewis structure for
is as follows:
j) Ion—
The number of valence electrons in
So, the Lewis structure for
is as follows:
k) Atom—
The number of valence electrons in
atom is
So, the Lewis structure for
is as follows:
l) Ion—
The number of valence electrons in
So, the Lewis structure for
is as follows:
m) Atom—
The number of valence electrons in
atom is
So, the Lewis structure for
is as follows:
n) Ion—
The number of valence electrons in
So, the Lewis structure for
is as follows:
Want to see more full solutions like this?
Chapter 8 Solutions
BURDGE CHEMISTRY VALUE ED (LL)
- Draw the mechanism for the substitution reaction converting an alcohol into an alkyl halide. If chirality is important to the reaction include it.arrow_forwardWrite, in words three different reactions we can use to make an alcohol.arrow_forwardDraw the reduction mechanism for the reduction of the aldehyde.arrow_forward
- What is the product of the reaction of XeF4 with H2O? Group of answer choices H2XeF2 H2XeF4 XeO3 H2XeOarrow_forwardWhile noble gas exerts the strongest London (dispersion) forces on neighboring atoms? Group of answer choices Xe Ar Kr Nearrow_forwardWhich of the following elements is corrosive to your skin due to that element breaking down C=C bonds? Group of answer choices fluorine iodine bromine chlorinearrow_forward
- What the best source of sulfide to use on a small scale in the lab? Group of answer choices thiourea H2S NaHS Na2Sarrow_forwardWhich of the following statements about sulfur is FALSE? Group of answer choices H2S is the product of an oxygen-depleted ecosystem. In the acid mine drainage reaction, FeS2 is a product. One allotrope of sulfur has the formula S20. In the environment, bacterial oxidation can convert S2− to elemental S or SO42−.arrow_forwardOf the following choices, which is the best reason that most materials DON'T spontaneously combust even though our atmosphere is about 21% oxygen? Group of answer choices The reduction of O2 in the gas phase (O2 + e− → O2−) is spontaneous. The reduction of O2 in acid solution (O2 + H+ + e− → HO2(aq)) is spontaneous. O2 is not a reactant in combustion. The O2 bond dissociation energy is 494 kJ/mol, leading to a high activation energy for combustion.arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
