
EBK CHEMISTRY FOR CHANGING TIMES
14th Edition
ISBN: 8220100663482
Author: MCCREARY
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Question
Chapter 8, Problem 10RQ
Interpretation Introduction
Interpretation:
Discharging process of a lead-storage battery should be explained.
Concept Introduction:
An
A battery is a device formed by a combination of electrochemical cells. It converts stored chemical energy into electricity.
A lead storage battery consists of lead plates immersed in sulfuric acid. Lead acid batteries contain a lead electrode and a lead oxide electrode dipped in sulfuric acid. When a lead storage battery discharges the following reaction occurs:
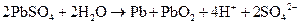
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Show work. Don't give Ai generated solution
Show reaction mechanism. Don't give Ai generated solution
Describe some isomerism that carboranes have.
Chapter 8 Solutions
EBK CHEMISTRY FOR CHANGING TIMES
Ch. 8 - Prob. 1RQCh. 8 - Prob. 2RQCh. 8 - Prob. 3RQCh. 8 - Prob. 4RQCh. 8 - Prob. 5RQCh. 8 - Prob. 6RQCh. 8 - Prob. 7RQCh. 8 - Prob. 8RQCh. 8 - Prob. 9RQCh. 8 - Prob. 10RQ
Ch. 8 - Prob. 11RQCh. 8 - Prob. 12RQCh. 8 - Prob. 13RQCh. 8 - Prob. 14RQCh. 8 - Prob. 15RQCh. 8 - Prob. 16PCh. 8 - Prob. 17PCh. 8 - Prob. 18PCh. 8 - Prob. 19PCh. 8 - 20. Write a balanced reduction half-reaction...Ch. 8 - Prob. 21PCh. 8 - Prob. 22PCh. 8 - Prob. 23PCh. 8 - Prob. 24PCh. 8 - Prob. 25PCh. 8 - Prob. 26PCh. 8 - Prob. 27PCh. 8 - Prob. 28PCh. 8 - Prob. 29PCh. 8 - Prob. 30PCh. 8 - Prob. 31PCh. 8 - Prob. 32PCh. 8 - Prob. 33PCh. 8 - Prob. 34PCh. 8 - Prob. 35PCh. 8 - Prob. 36PCh. 8 - Prob. 37PCh. 8 - Prob. 38PCh. 8 - Prob. 39PCh. 8 - Prob. 40PCh. 8 - Prob. 41PCh. 8 - Prob. 42PCh. 8 - Prob. 43PCh. 8 - 44. Tantalum, a metal used m electronic devices...Ch. 8 - Prob. 45PCh. 8 - Prob. 46PCh. 8 - Prob. 47PCh. 8 - Prob. 48PCh. 8 - Prob. 49PCh. 8 - Prob. 50PCh. 8 - Prob. 51PCh. 8 - Prob. 52PCh. 8 - Prob. 53PCh. 8 - Prob. 54PCh. 8 - Prob. 55APCh. 8 - Prob. 56APCh. 8 - Prob. 57APCh. 8 - Prob. 58APCh. 8 - Prob. 59APCh. 8 - Prob. 60APCh. 8 - Prob. 61APCh. 8 - When exposed to air containing hydrogen sulfide,...Ch. 8 - Prob. 63APCh. 8 - Prob. 64APCh. 8 - Prob. 65APCh. 8 - Prob. 66APCh. 8 - Prob. 67APCh. 8 - Prob. 68APCh. 8 - Prob. 69APCh. 8 - Prob. 70APCh. 8 - Prob. 71APCh. 8 - Prob. 72APCh. 8 - Prob. 73APCh. 8 - Prob. 74APCh. 8 - Prob. 75APCh. 8 - Prob. 76APCh. 8 - Prob. 77APCh. 8 - Prob. 78APCh. 8 - Prob. 79APCh. 8 - Prob. 80APCh. 8 - Prob. 8.1CTECh. 8 - Prob. 8.2CTECh. 8 - Prob. 8.3CTECh. 8 - Prob. 8.4CTECh. 8 - Prob. 8.5CTECh. 8 - Prob. 8.6CTECh. 8 - Prob. 8.7CTECh. 8 - Prob. 1CGPCh. 8 - Prob. 2CGPCh. 8 - Prob. 3CGPCh. 8 - Prob. 4CGPCh. 8 - Prob. 5CGPCh. 8 - Prob. 1CHQCh. 8 - Prob. 2CHQCh. 8 - Prob. 3CHQCh. 8 - Prob. 4CHQCh. 8 - Prob. 5CHQ
Knowledge Booster
Similar questions
- Nonearrow_forwardTransmitance 3. Which one of the following compounds corresponds to this IR spectrum? Point out the absorption band(s) that helped you decide. OH H3C OH H₂C CH3 H3C CH3 H3C INFRARED SPECTRUM 0.8- 0.6 0.4- 0.2 3000 2000 1000 Wavenumber (cm-1) 4. Consider this compound: H3C On the structure above, label the different types of H's as A, B, C, etc. In table form, list the labeled signals, and for each one state the number of hydrogens, their shifts, and the splitting you would observe for these hydrogens in the ¹H NMR spectrum. Label # of hydrogens splitting Shift (2)arrow_forwardNonearrow_forward
- Draw the Lewis structure of C2H4Oarrow_forwarda) 5. Circle all acidic (and anticoplanar to the Leaving group) protons in the following molecules, Solve these elimination reactions, and identify the major and minor products where appropriate: 20 points + NaOCH3 Br (2 productarrow_forwardNonearrow_forward
- Dr. Mendel asked his BIOL 260 class what their height was and what their parent's heights were. He plotted that data in the graph below to determine if height was a heritable trait. A. Is height a heritable trait? If yes, what is the heritability value? (2 pts) B. If the phenotypic variation is 30, what is the variation due to additive alleles? (2 pts) Offspring Height (Inches) 75 67.5 60 52.5 y = 0.9264x + 4.8519 55 60 65 MidParent Height (Inches) 70 75 12pt v V Paragraph B IUA > AT2 v Varrow_forwardExperiment: Each team will be provided with 5g of a mixture of acetanilide and salicylic acid. You will divide it into three 1.5 g portions in separate 125 mL Erlenmeyer flasks savıng some for melting point analysis. Dissolve the mixture in each flask in ~60mL of DI water by heating to boiling on a hotplate. Take the flasks off the hotplate once you have a clear solution and let them stand on the bench top for 5 mins and then allow them to cool as described below. Sample A-Let the first sample cool slowly to room temperature by letting it stand on your lab bench, with occasional stirring to promote crystallization. Sample B-Cool the second sample 1n a tap-water bath to 10-15 °C Sample C-Cool the third sample in an ice-bath to 0-2 °C Results: weight after recrystalization and melting point temp. A=0.624g,102-115° B=0.765g, 80-105° C=1.135g, 77-108 What is the percent yield of A,B, and C.arrow_forwardRel. Intensity Q 1. Which one of the following is true of the compound whose mass spectrum is shown here? Explain how you decided. 100 a) It contains chlorine. b) It contains bromine. c) It contains neither chlorine nor bromine. 80- 60- 40- 20- 0.0 0.0 TT 40 80 120 160 m/z 2. Using the Table of IR Absorptions how could you distinguish between these two compounds in the IR? What absorbance would one compound have that the other compound does not? HO CIarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY