
a)
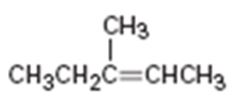
Interpretation:
Assuming Markovnikov’s rule is valid, the major alcohol product obtainable by the acid-catalyzed addition of water to 3-methyl-3-butene is to be predicted.
Concept introduction:
Markovnikov’s rule can be used to decide on the orientation in electrophilic addition reactions. According to this rule, in the addition of HX to an
To state:
The major alcohol product obtainable by the acid-catalyzed addition of water to 3-methyl-3-butene assuming that Markovnikov’s rule is valid.
b)
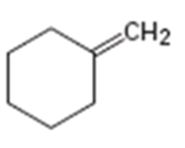
Interpretation:
Assuming Markovnikov’s rule is valid, the major alcohol product obtainable by the acid-catalyzed addition of water to methylidenecyclohexene is to be predicted.
Concept introduction:
Markovnikov’s rule can be used to decide on the orientation in electrophilic addition reactions. According to this rule, in the addition of HX to an alkene, the H attaches itself with the carbon with fewer alkyl substituents and the X attaches itself with the carbon with more alkyl substituents.
To state:
The major alcohol product obtainable by the acid-catalyzed addition of water to methylidenecyclohexene assuming that Markovnikov’s rule is valid.
c)
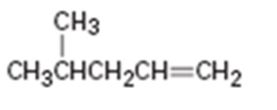
Interpretation:
Assuming Markovnikov’s rule is valid, the major alcohol product obtainable by the acid-catalyzed addition of water to 4- methyl-1-pentene is to be predicted.
Concept introduction:
Markovnikov’s rule can be used to decide on the orientation in electrophilic addition reactions. According to this rule, in the addition of HX to an alkene, the H attaches itself with the carbon with fewer alkyl substituents and the X attaches itself with the carbon with more alkyl substituents.
To state:
The major alcohol product obtainable by the acid-catalyzed addition of water to 4- methyl-1-pentene assuming that Markovnikov’s rule is valid.
Trending nowThis is a popular solution!

Chapter 7 Solutions
EP ORGANIC CHEMISTRY,24 MONTH-OWLV2
- Draw an example of the following functional groups: *see imagearrow_forwardAldehydes and Ketones: Show the reaction conditions, and molecules, that connect the reactant to the product. A protecting group will be needed. *see imagearrow_forwardAldehydes and Ketones: Show the reaction conditions, and molecules, that connect the reactant to the product. *see imagearrow_forward
- Provide the missing information for each of the four reactions: *see imagearrow_forward6. Chlorine dioxide (CIO) is used as a disinfectant in municipal water-treatment plants. It decomposes in a first-order reaction with a rate constant of 14 s. How long would it take for an initial concentration of 0.06 M to decrease to 0.02 M? [6 pts]arrow_forwardIf possible, replace an H atom on the a carbon of the molecule in the drawing area with a methyl group substituent, and replace an H atom on the ẞ carbon with a hydroxyl group substituent. If one of the substituents can't be added for any reason, just don't add it. If neither substituent can be added, check the box under the drawing area. en HO OHarrow_forward
- Curved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the curved arrows to draw the intermediate and product of this hydrohalogenation reaction. Include all lone pairs and charges as appropriate. Br Select to Draw 51°F Sunny esc F1 HBr Select to Draw 1,2-hydride shift Br Select to Draw Q Search F2 F3 F4 1 2 # # 3 DII L F5 F6 F tA $ % Λarrow_forwardplease help i cant find the article to even startarrow_forwardWhat are the missing reagents for the spots labeled 1 and 3? Please give a detailed explanation and include the drawings and show how the synthesis proceeds with the reagents.arrow_forward
- help with the rf values i am so confusedarrow_forwardPredict the organic reactant of X and Y that are involved in the reaction below, and draw the skeletal ("line") structures of the missing organic reactant. Please include all steps & drawings & explanations.arrow_forwardPredict the major organic product for this reaction.arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


