
Introduction to General, Organic and Biochemistry
11th Edition
ISBN: 9781285869759
Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 7.7, Problem 7.7P
Problem 7-7
Consider the following equilibrium reaction for the decomposition of an aqueous solution of hydrogen peroxide:
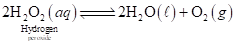
Oxygen has limited solubility in water (see the table in Chemical Connections 6A). What happens to the equilibrium after the solution becomes saturated with oxygen?
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Assign these proton
Could you please solve the first problem in this way and present it similarly but color-coded or step by step so I can understand it better? Thank you!
Could you please solve the first problem in this way and present it similarly but color-coded or step by step so I can understand it better? Thank you!
Chapter 7 Solutions
Introduction to General, Organic and Biochemistry
Ch. 7.1 - Problem 7-1 In the reaction we measure the...Ch. 7.4 - Problem 7-2 Calculate the rate for the reaction in...Ch. 7.6 - Prob. 7.3PCh. 7.6 - Prob. 7.4PCh. 7.6 - Prob. 7.5PCh. 7.7 - Prob. 7.6PCh. 7.7 - Problem 7-7 Consider the following equilibrium...Ch. 7.7 - Prob. 7.8PCh. 7.7 - Prob. 7.9PCh. 7 - 7-10 The rate of disappearance of HCI was measured...
Ch. 7 - 7-11 Consider the following reaction: Suppose we...Ch. 7 - 7-12 Two kinds of gas molecules are reacted at a...Ch. 7 - 7-13 Why are reactions between ions in aqueous...Ch. 7 - Prob. 7.14PCh. 7 - 7-15 A certain reaction is exothermic by 9...Ch. 7 - 7-16 A quart of milk quickly spoils if left at...Ch. 7 - 7-17 If a certain reaction takes 16 h to go to...Ch. 7 - Prob. 7.18PCh. 7 - Prob. 7.19PCh. 7 - Prob. 7.20PCh. 7 - Prob. 7.21PCh. 7 - 7-22 If you add a piece of marble, CaCO3 to a 6 M...Ch. 7 - Prob. 7.23PCh. 7 - Prob. 7.24PCh. 7 - Prob. 7.25PCh. 7 - 7-26 Write the chemical equations corresponding to...Ch. 7 - Prob. 7.27PCh. 7 - 7-28 When the following reaction reached...Ch. 7 - 7-29 The following reaction was allowed to reach...Ch. 7 - Prob. 7.30PCh. 7 - 7-31 Here are equilibrium constants for several...Ch. 7 - 7-32 A particular reaction has an equilibrium...Ch. 7 - Prob. 7.33PCh. 7 - Prob. 7.34PCh. 7 - 7-35 A reaction has a high rate constant but a...Ch. 7 - 7-36 Complete the following table showing the...Ch. 7 - Prob. 7.37PCh. 7 - Prob. 7.38PCh. 7 - Prob. 7.39PCh. 7 - 7-40 Is there any change in conditions that change...Ch. 7 - 7-41 The equilibrium constant at 1127°C for the...Ch. 7 - Prob. 7.42PCh. 7 - 7-43 (Chemical Connections 7A and 7B) Why is a...Ch. 7 - Prob. 7.44PCh. 7 - 7-45 (Chemical Connections 7C) A painkiller—for...Ch. 7 - 7-46 (Chemical Connections 7D) What reaction takes...Ch. 7 - Prob. 7.47PCh. 7 - Prob. 7.48PCh. 7 - Prob. 7.49PCh. 7 - 7-50 Draw an energy diagram for an exothermic...Ch. 7 - Prob. 7.51PCh. 7 - Prob. 7.52PCh. 7 - Prob. 7.53PCh. 7 - Prob. 7.54PCh. 7 - Prob. 7.55PCh. 7 - Prob. 7.56PCh. 7 - 7-57 Write the reaction to which the following...Ch. 7 - Prob. 7.58PCh. 7 - Prob. 7.59PCh. 7 - Prob. 7.60PCh. 7 - Prob. 7.61PCh. 7 - Prob. 7.62PCh. 7 - Prob. 7.63PCh. 7 - 7-64 As we shall see in Chapter 20, there are two...Ch. 7 - Prob. 7.65PCh. 7 - Prob. 7.66PCh. 7 - Prob. 7.67PCh. 7 - Prob. 7.68PCh. 7 - 7-69 Pure carbon exists is several forms, two of...Ch. 7 - Prob. 7.70PCh. 7 - 7-71 You have a beaker that contains solid silver...Ch. 7 - Prob. 7.72PCh. 7 - Prob. 7.73PCh. 7 - Prob. 7.74PCh. 7 - Prob. 7.75PCh. 7 - Prob. 7.76PCh. 7 - Prob. 7.77PCh. 7 - Prob. 7.78PCh. 7 - Prob. 7.79PCh. 7 - Prob. 7.80PCh. 7 - Prob. 7.81PCh. 7 - 7-82 An equilibrium mixture of O2, SO2, and SO3...Ch. 7 - Prob. 7.83PCh. 7 - Prob. 7.84P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Could you please solve the first problem in this way and present it similarly but (color-coded) and step by step so I can understand it better? Thank you! I want to see what they are doingarrow_forwardCan you please help mne with this problem. Im a visual person, so can you redraw it, potentislly color code and then as well explain it. I know im given CO2 use that to explain to me, as well as maybe give me a second example just to clarify even more with drawings (visuals) and explanations.arrow_forwardPart 1. Aqueous 0.010M AgNO 3 is slowly added to a 50-ml solution containing both carbonate [co32-] = 0.105 M and sulfate [soy] = 0.164 M anions. Given the ksp of Ag2CO3 and Ag₂ soy below. Answer the ff: Ag₂ CO3 = 2 Ag+ caq) + co} (aq) ksp = 8.10 × 10-12 Ag₂SO4 = 2Ag+(aq) + soy² (aq) ksp = 1.20 × 10-5 a) which salt will precipitate first? (b) What % of the first anion precipitated will remain in the solution. by the time the second anion starts to precipitate? (c) What is the effect of low pH (more acidic) condition on the separate of the carbonate and sulfate anions via silver precipitation? What is the effect of high pH (more basic)? Provide appropriate explanation per answerarrow_forward
- Part 4. Butanoic acid (ka= 1.52× 10-5) has a partition coefficient of 3.0 (favors benzene) when distributed bet. water and benzene. What is the formal concentration of butanoic acid in each phase when 0.10M aqueous butanoic acid is extracted w❘ 25 mL of benzene 100 mL of a) at pit 5.00 b) at pH 9.00arrow_forwardCalculate activation energy (Ea) from the following kinetic data: Temp (oC) Time (s) 23.0 180. 32.1 131 40.0 101 51.8 86.0 Group of answer choices 0.0269 kJ/mole 2610 kJ/mole 27.6 kJ/mole 0.215 kJ/mole 20.8 kJ/molearrow_forwardCalculate activation energy (Ea) from the following kinetic data: Temp (oC) Time (s) 23.0 180. 32.1 131 40.0 101 51.8 86.0 choices: 0.0269 kJ/mole 2610 kJ/mole 27.6 kJ/mole 0.215 kJ/mole 20.8 kJ/molearrow_forward
- Determine the rate law for sodium thiosulfate from the following data: [Na2S2O3] Time (s) 0.0318 230. 0.0636 57.5arrow_forwardPlease solvearrow_forwardRank the compounds in each group below according to their reactivity toward electrophilic aromatic substitution (most reactive = 1; least reactive = 3). Place the number corresponding to the compounds' relative reactivity in the blank below the compound. a. CH₂F CH3 F b. At what position, and on what ring, is bromination of phenyl benzoate expected to occur? Explain your answer. :0: C-O phenyl benzoate 6.Consider the reaction below to answer the following questions. A B C NO₂ FeBr3 + Br₂ D a. The nucleophile in the reaction is: BODADES b. The Lewis acid catalyst in the reaction is: C. This reaction proceeds d. Draw the structure of product D. (faster or slower) than benzene.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning
Kinetics: Chemistry's Demolition Derby - Crash Course Chemistry #32; Author: Crash Course;https://www.youtube.com/watch?v=7qOFtL3VEBc;License: Standard YouTube License, CC-BY