
7-64 As we shall see in Chapter 20, there are two forms of glucose, designated alpha 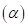 and beta
and beta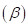 which are in equilibrium in aqueous solution. The equilibrium constant for the reaction is 1.5 at 30°C.
which are in equilibrium in aqueous solution. The equilibrium constant for the reaction is 1.5 at 30°C.
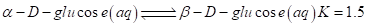
(a) If you begin with a fresh 1.0 M solution of  D-glucose in water, what will be its concentration when equilibrium is reached?
D-glucose in water, what will be its concentration when equilibrium is reached?
(b) Calculate the percentage of 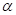 glucose and of
glucose and of 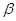 glucose present at equilibrium in aqueous solution at 30°C.
glucose present at equilibrium in aqueous solution at 30°C.
(a)
Interpretation:
The equilibrium concentrations of
Concept Introduction:
Answer to Problem 7.64P
Explanation of Solution
The given equilibrium reaction is,
The initial concentration of
Setting up the equilibrium changes to calculate the equilibrium concentrations:
Therefore,
(b)
Interpretation:
The percentages of
Concept Introduction:
Answer to Problem 7.64P
Percentage of
Percentage of
Explanation of Solution
The given equilibrium reaction is,
The initial concentration of
From the above calculations, concentration of
Percentage of
Percentage of
Want to see more full solutions like this?
Chapter 7 Solutions
Introduction to General, Organic and Biochemistry
- Which of the following molecules are NOT typical carbohydrates? For the molecules that are carbohydrates, label them as an aldose or ketose. HO Он ОН ОН Он ОН но ΤΗ HO ОН HO eve Он он ОН ОН ОН If polyethylene has an average molecular weight of 25,000 g/mol, how many repeat units are present?arrow_forwardDraw the a-anomer cyclized pyranose Haworth projection of the below hexose. Circle the anomeric carbons. Number the carbons on the Fischer and Haworth projections. Assign R and S for each chiral center. HO CHO -H HO -H H- -OH H -OH CH₂OH Draw the ẞ-anomer cyclized furanose Haworth projection for the below hexose. Circle the anomeric carbons. Number the carbons on the Fischer and Haworth projections. HO CHO -H H -OH HO -H H -OH CH₂OHarrow_forwardName the below disaccharide. Circle any hemiacetals. Identify the numbering of glycosidic linkage, and identify it as a or ẞ. OH HO HO OH HO HO HO OHarrow_forward
- What are the monomers used to make the following polymers? F. а. b. с. d. Вецер хочому なarrow_forward1. Propose a reasonable mechanism for the following transformation. I'm looking for curved mechanistic arrows and appropriate formal charges on intermediates. OMe MeO OMe Me2N NMe2 OTBS OH xylenes OMe 'OTBSarrow_forwardWhat is the polymer made from the following monomers? What type of polymerization is used for each? а. ОН H2N но b. ن -NH2 d. H₂N NH2 довarrow_forward
- Condensation polymers are produced when monomers containing two different functional groups link together with the loss of a small molecule such as H2O. The difunctional monomer H2N(CH2)6COOH forms a condensation polymer. Draw the carbon-skeleton structure of the dimer that forms from this monomer.arrow_forwardWhat is the structure of the monomer?arrow_forward→ BINDERIYA GANBO... BINDERIYA GANBO. AP Biology Notes Gamino acid chart - G... 36:22 司 10 ☐ Mark for Review Q 1 Hide 80 8 2 =HA O=A¯ = H₂O Acid HIO HBrO HCIO Question 10 of 35 ^ Σ DELL □ 3 % Λ & 6 7 * ∞ 8 do 5 $ 4 # m 3 ° ( 9 Highlights & Notes AXC Sign out Carrow_forward
- Which representation(s) show polymer structures that are likely to result in rigid, hard materials and those that are likely to result in flexible, stretchable, soft materials?arrow_forward3. Enter the molecular weight of the product obtained from the Williamson Ether Synthesis? OH OH & OH excess CH3l Ag₂Oarrow_forwardPlease answer 1, 2 and 3 on the endarrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





