
Organic Chemistry As a Second Language: Second Semester Topics
4th Edition
ISBN: 9781119110651
Author: David R. Klein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 7.6, Problem 7.37P
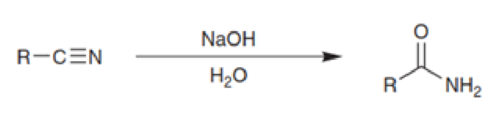
Based on everything we have just seen, propose a mechanism for the hydration of a nitrile under basic conditions:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Provide the missing information. *see image
Why does the following reaction lead to poor yields? Correct the reaction. *see image
Provide the missing information. *see image
Chapter 7 Solutions
Organic Chemistry As a Second Language: Second Semester Topics
Ch. 7.3 - Propose a plausible mechanism for each of the...Ch. 7.3 - Propose a plausible mechanism for each of the...Ch. 7.3 - Propose a plausible mechanism for each of the...Ch. 7.3 - Propose a plausible mechanism for each of the...Ch. 7.3 - Propose a plausible mechanism for each of the...Ch. 7.3 - Predict the major product in each of the following...Ch. 7.3 - Predict the major product in each of the following...Ch. 7.3 - Predict the major product in each of the following...Ch. 7.3 - Predict the major product in each of the following...Ch. 7.3 - Predict the major product in each of the following...
Ch. 7.3 - Identify the reagents you would use to achieve...Ch. 7.3 - Identify the reagents you would use to achieve...Ch. 7.3 - Identify the reagents you would use to achieve...Ch. 7.3 - Identify the reagents you would use to achieve...Ch. 7.3 - Identify the reagents you would use to achieve...Ch. 7.4 - Predict the major products for each of the...Ch. 7.4 - Predict the major products for each of the...Ch. 7.4 - Predict the major products for each of the...Ch. 7.5 - Prob. 7.23PCh. 7.5 - Identify the reagents you would use to make each...Ch. 7.5 - Identify the reagents you would use to make each...Ch. 7.5 - Identify the reagents you would use to make each...Ch. 7.5 - Identify the reagents you would use to make each...Ch. 7.5 - In the space provided, draw a mechanism for each...Ch. 7.5 - In the space provided, draw a mechanism for each...Ch. 7.5 - Prob. 7.33PCh. 7.5 - Prob. 7.34PCh. 7.5 - Prob. 7.35PCh. 7.5 - Prob. 7.36PCh. 7.6 - Based on everything we have just seen, propose a...Ch. 7.6 - Prob. 7.39PCh. 7.6 - Prob. 7.40PCh. 7.6 - Prob. 7.41PCh. 7.6 - Propose a mechanism for the following reaction:Ch. 7.6 - Prob. 7.44PCh. 7.6 - Prob. 7.45PCh. 7.6 - Prob. 7.46PCh. 7.7 - Prob. 7.48PCh. 7.7 - Prob. 7.49PCh. 7.7 - Prob. 7.50PCh. 7.7 - Prob. 7.51PCh. 7.7 - Prob. 7.52PCh. 7.7 - Prob. 7.53PCh. 7.7 - Prob. 7.55PCh. 7.7 - Prob. 7.56PCh. 7.7 - Prob. 7.57PCh. 7.7 - Prob. 7.58PCh. 7.7 - Prob. 7.59PCh. 7.7 - Prob. 7.60PCh. 7.7 - Prob. 7.61PCh. 7.7 - Prob. 7.62PCh. 7.7 - Prob. 7.63PCh. 7.7 - Prob. 7.64PCh. 7.7 - Prob. 7.65P
Additional Science Textbook Solutions
Find more solutions based on key concepts
What is the difference between cellular respiration and external respiration?
Human Physiology: An Integrated Approach (8th Edition)
WRITE ABOUT A THEME: ORGANIZATION Cells arc the basic units of structure and function in all organisms. A key f...
Campbell Biology (11th Edition)
PRACTICE 1.3 The melting point of table salt is 1474oF. What temperature is this on the Celsius and Kelvin scal...
Chemistry (7th Edition)
41. A reaction in which A, B, and C react to form products is first order in A, second order in B, and zero ord...
Chemistry: Structure and Properties (2nd Edition)
Practice Problem 2.11 Although we shall discuss the naming of organic compounds later when we discuss the indiv...
Organic Chemistry
Flask A contains yeast cells in glucose-minimal salts broth incubated at 30C with aeration. Flask B contains ye...
Microbiology: An Introduction
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the mechanism (including all curved arrows for electron movement) showing how the maleicanhydride is attacked by the anthracene and formation of the final Diels Alder product.arrow_forwardProvide the missing information. *see imagearrow_forwardProvide the missing information. *see imagearrow_forward
- Provide the missing information. *see imagearrow_forwardI have a bottle of butanal that has been improperly used by lab workers. They allowed a traceamount NaOH (aq) to contaminate the bottle. What is now in my bottle of “butanal? What is the molecular name and functional group name? Draw the structure.arrow_forwardProvide the missing information. *see imagearrow_forward
- First image: Why can't the molecule C be formed in those conditions Second image: Synthesis for lactone C its not an examarrow_forwardFirst image: I have to show the mecanism for the reaction on the left, where the alcohol A is added fast in one portion Second image: I have to show the mecanism of the reaction at the bottom. Also I have to show by mecanism why the reaction wouldn't work if the alcohol was primaryarrow_forwardFirst image: I have to explain why the molecule C is never formed in those conditions. Second image: I have to propose a synthesis for the lactone Aarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning


Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole
Coenzymes and cofactors; Author: CH15 SWAYAM Prabha IIT Madras;https://www.youtube.com/watch?v=bubY2Nm7hVM;License: Standard YouTube License, CC-BY
Aromaticity and Huckel's Rule; Author: Professor Dave Explains;https://www.youtube.com/watch?v=7-BguH4_WBQ;License: Standard Youtube License