
Organic Chemistry As a Second Language: Second Semester Topics
4th Edition
ISBN: 9781119110651
Author: David R. Klein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 7.4, Problem 7.21P
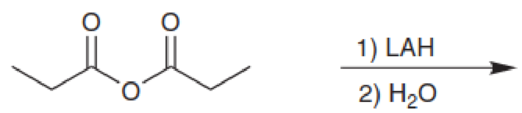
Predict the major products for each of the following reactions:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
C.
Ν
H
a.
H3C.
N
H3C
CH3
HCN
ол
2.
восцапан
(46:00)
Curtius rearrangment
1. NaN3, heat
-OH
Chapter 7 Solutions
Organic Chemistry As a Second Language: Second Semester Topics
Ch. 7.3 - Propose a plausible mechanism for each of the...Ch. 7.3 - Propose a plausible mechanism for each of the...Ch. 7.3 - Propose a plausible mechanism for each of the...Ch. 7.3 - Propose a plausible mechanism for each of the...Ch. 7.3 - Propose a plausible mechanism for each of the...Ch. 7.3 - Predict the major product in each of the following...Ch. 7.3 - Predict the major product in each of the following...Ch. 7.3 - Predict the major product in each of the following...Ch. 7.3 - Predict the major product in each of the following...Ch. 7.3 - Predict the major product in each of the following...
Ch. 7.3 - Identify the reagents you would use to achieve...Ch. 7.3 - Identify the reagents you would use to achieve...Ch. 7.3 - Identify the reagents you would use to achieve...Ch. 7.3 - Identify the reagents you would use to achieve...Ch. 7.3 - Identify the reagents you would use to achieve...Ch. 7.4 - Predict the major products for each of the...Ch. 7.4 - Predict the major products for each of the...Ch. 7.4 - Predict the major products for each of the...Ch. 7.5 - Prob. 7.23PCh. 7.5 - Identify the reagents you would use to make each...Ch. 7.5 - Identify the reagents you would use to make each...Ch. 7.5 - Identify the reagents you would use to make each...Ch. 7.5 - Identify the reagents you would use to make each...Ch. 7.5 - In the space provided, draw a mechanism for each...Ch. 7.5 - In the space provided, draw a mechanism for each...Ch. 7.5 - Prob. 7.33PCh. 7.5 - Prob. 7.34PCh. 7.5 - Prob. 7.35PCh. 7.5 - Prob. 7.36PCh. 7.6 - Based on everything we have just seen, propose a...Ch. 7.6 - Prob. 7.39PCh. 7.6 - Prob. 7.40PCh. 7.6 - Prob. 7.41PCh. 7.6 - Propose a mechanism for the following reaction:Ch. 7.6 - Prob. 7.44PCh. 7.6 - Prob. 7.45PCh. 7.6 - Prob. 7.46PCh. 7.7 - Prob. 7.48PCh. 7.7 - Prob. 7.49PCh. 7.7 - Prob. 7.50PCh. 7.7 - Prob. 7.51PCh. 7.7 - Prob. 7.52PCh. 7.7 - Prob. 7.53PCh. 7.7 - Prob. 7.55PCh. 7.7 - Prob. 7.56PCh. 7.7 - Prob. 7.57PCh. 7.7 - Prob. 7.58PCh. 7.7 - Prob. 7.59PCh. 7.7 - Prob. 7.60PCh. 7.7 - Prob. 7.61PCh. 7.7 - Prob. 7.62PCh. 7.7 - Prob. 7.63PCh. 7.7 - Prob. 7.64PCh. 7.7 - Prob. 7.65P
Additional Science Textbook Solutions
Find more solutions based on key concepts
Define histology.
Fundamentals of Anatomy & Physiology (11th Edition)
What are four functions of connective tissue?
Anatomy & Physiology (6th Edition)
12.1 Give the IUPAC name for each of the following:
a. CH3-CH2-OH
b.
c.
d.
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Practice Exercise 2
Aspirin is composed of 60.0% carbon, 4.5% hydrogen, and 35.5% oxygen by mass, regardless o...
Chemistry: The Central Science (14th Edition)
Community 1 contains 100 individuals distributed among four species: 5A, 5B, 85C, and 5D. Community 2 contains ...
Campbell Biology (11th Edition)
All of the following processes are involved in the carbon cycle except: a. photosynthesis b. cell respiration c...
Human Biology: Concepts and Current Issues (8th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Question 1. Please predict the products for each of the following reactions. Clearly show the regiochemistry (Markovnikov vs anti-Markovnikov) and stereochemistry (syn- vs anti- or both). If a mixture of enantiomers is formed, please draw all the enantiomers.arrow_forwardElectrochemistry. Briefly describe the Donnan potential.arrow_forwardIndicate what the Luther equation is used for?arrow_forward
- Indicate one aspect that benefits and another that makes it difficult to use the hydroquinone electrode to measure pH.arrow_forwardAt an electrified interface according to the Gouy-Chapman model, what types of interactions do NOT occur between the ions and the solvent according to this theory?arrow_forwardPlease predict the products for each of the following reactions. Clearly show the regiochemistry (Markovnikov vs anti-Markovnikov) and stereochemistry (syn- vs anti- or both). If a mixture of enantiomers is formed, please draw all the enantiomers. Hint: In this case you must choose the best answer to demonstrate the stereochemistry of H2 addition. 1.03 2. (CH3)2S BIZ CH₂OH 2. DMS KMnO4, NaOH ΖΗ Pd or Pt (catalyst) HBr 20 1 HBr ROOR (peroxide) HO H-SO HC 12 11 10 BH, THE 2. H2O2, NaOH Brz cold HI 19 18 17 16 MCPBA 15 14 13 A Br H₂O BH3⚫THF Brz EtOH Pd or Ni (catalyst) D₂ (deuterium) 1. Os04 2. H2O2 CH3CO3H (peroxyacid) 1. MCPBA 2. H₂O* H B + H H H "H C H H Darrow_forward
- Explain how Beer’s Law can be used to determine the concentration in a selected food sample. Provide examples.arrow_forwardExplain the importance of having a sampling plan with respect to food analysis. Explain the importance of having a sampling plan with respect to food analysis. Provide examples.arrow_forwardPlease predict the products for each of the following reactions. Clearly show the regiochemistry (Markovnikov vs anti-Markovnikov) and stereochemistry (syn- vs anti- or both). If a mixture of enantiomers is formed, please draw all the enantiomers. cold KMnO4, NaOH 2. DMS 1. 03 CH3OH Br2 1. 03 2. (CH3)2S H₂ Pd or Pt (catalyst) HBr 18 19 20 1 HBr ROOR (peroxide) H₂O H₂SO4 HCI HI 17 16 6 15 MCPBA 1. BH3 THF 2. H₂O2, NaOH 1. OsO4 2. H₂O₂ 110 CH3CO₂H (peroxyacid) 1. MCPBA 2. H₂O* Br2 H₂O BH3 THF B12 EtOH Pd or Ni (catalyst) D₂ (deuterium) Bra A B C D H OH H OH OH H OH α α α OH H OH OH фон d H "Harrow_forward
- Briefly indicate the models that describe the structure of the interface: Helmholtz-Perrin, Gouy-Chapman, Stern and Grahame models.arrow_forwardElectrochemistry. Briefly describe the Gibbs model and the Gibbs absorption equation.arrow_forwardThermodynamic analysis of electrified interfaces.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

Characteristic Reactions of Benzene and Phenols; Author: Linda Hanson;https://www.youtube.com/watch?v=tjEqEjDd87E;License: Standard YouTube License, CC-BY
An Overview of Aldehydes and Ketones: Crash Course Organic Chemistry #27; Author: Crash Course;https://www.youtube.com/watch?v=-fBPX-4kFlw;License: Standard Youtube License