
Organic Chemistry As a Second Language: Second Semester Topics
4th Edition
ISBN: 9781119110651
Author: David R. Klein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 7.3, Problem 7.3P
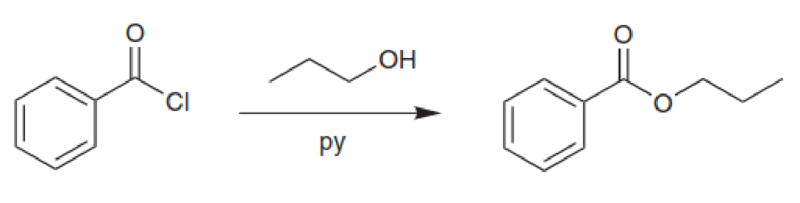
Propose a plausible mechanism for each of the following reactions. Space has been provided for you to record your answer directly below each reaction:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
None
None
Please correct answer and don't use hand rating
Chapter 7 Solutions
Organic Chemistry As a Second Language: Second Semester Topics
Ch. 7.3 - Propose a plausible mechanism for each of the...Ch. 7.3 - Propose a plausible mechanism for each of the...Ch. 7.3 - Propose a plausible mechanism for each of the...Ch. 7.3 - Propose a plausible mechanism for each of the...Ch. 7.3 - Propose a plausible mechanism for each of the...Ch. 7.3 - Predict the major product in each of the following...Ch. 7.3 - Predict the major product in each of the following...Ch. 7.3 - Predict the major product in each of the following...Ch. 7.3 - Predict the major product in each of the following...Ch. 7.3 - Predict the major product in each of the following...
Ch. 7.3 - Identify the reagents you would use to achieve...Ch. 7.3 - Identify the reagents you would use to achieve...Ch. 7.3 - Identify the reagents you would use to achieve...Ch. 7.3 - Identify the reagents you would use to achieve...Ch. 7.3 - Identify the reagents you would use to achieve...Ch. 7.4 - Predict the major products for each of the...Ch. 7.4 - Predict the major products for each of the...Ch. 7.4 - Predict the major products for each of the...Ch. 7.5 - Prob. 7.23PCh. 7.5 - Identify the reagents you would use to make each...Ch. 7.5 - Identify the reagents you would use to make each...Ch. 7.5 - Identify the reagents you would use to make each...Ch. 7.5 - Identify the reagents you would use to make each...Ch. 7.5 - In the space provided, draw a mechanism for each...Ch. 7.5 - In the space provided, draw a mechanism for each...Ch. 7.5 - Prob. 7.33PCh. 7.5 - Prob. 7.34PCh. 7.5 - Prob. 7.35PCh. 7.5 - Prob. 7.36PCh. 7.6 - Based on everything we have just seen, propose a...Ch. 7.6 - Prob. 7.39PCh. 7.6 - Prob. 7.40PCh. 7.6 - Prob. 7.41PCh. 7.6 - Propose a mechanism for the following reaction:Ch. 7.6 - Prob. 7.44PCh. 7.6 - Prob. 7.45PCh. 7.6 - Prob. 7.46PCh. 7.7 - Prob. 7.48PCh. 7.7 - Prob. 7.49PCh. 7.7 - Prob. 7.50PCh. 7.7 - Prob. 7.51PCh. 7.7 - Prob. 7.52PCh. 7.7 - Prob. 7.53PCh. 7.7 - Prob. 7.55PCh. 7.7 - Prob. 7.56PCh. 7.7 - Prob. 7.57PCh. 7.7 - Prob. 7.58PCh. 7.7 - Prob. 7.59PCh. 7.7 - Prob. 7.60PCh. 7.7 - Prob. 7.61PCh. 7.7 - Prob. 7.62PCh. 7.7 - Prob. 7.63PCh. 7.7 - Prob. 7.64PCh. 7.7 - Prob. 7.65P
Additional Science Textbook Solutions
Find more solutions based on key concepts
Q2. Which statement best defines chemistry?
a. The science that studies solvents, drugs, and insecticides
b. Th...
Introductory Chemistry (6th Edition)
61. What is the pH of a solution in which 224 mL of HCl(g), measured at 27.2 °C and 1.02 atm, is dissolved in 1...
Chemistry: A Molecular Approach (4th Edition)
Explain why 92% of 2,4-pemtanedione exists as the enol tautomer in hexane but only 15% of this compound exists ...
Organic Chemistry (8th Edition)
45. Calculate the mass of nitrogen dissolved at room temperature in an 80.0-L home aquarium. Assume a total pre...
Chemistry: Structure and Properties (2nd Edition)
Why are BSL-4 suits pressurized? Why not just wear tough regular suits?
Microbiology with Diseases by Body System (5th Edition)
68. A spaceship of mass 2.0 × 106 kg is cruising at a speed of 5.0 × 106 m/s when the antimatter reactor fails,...
College Physics: A Strategic Approach (3rd Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Nonearrow_forwardDraw Newman projects for each of the following molecules with 3 different rotational angles from carbon 2 to carbon 3. Rank your structures from lowest to highest energy. What causes the energy differences? Label the overlap. a. b. Br OH C. Br Brarrow_forwardDraw the stereoisomers of 3,5-diethylcylopentane. Identify the different relationships between each molecules (diasteromers, enantiomers, meso compounds, etc.)arrow_forward
- Is it possible to do the following reduction in one step? If so, add the necessary reagents and catalysts to the reaction arrow. If not, check the box under the drawing area. T G टे 13arrow_forwardPlease correct answer and don't use hand ratingarrow_forward2. Draw mechanisms for the following reactions. mg Et CO₂Hot H30t Et 0arrow_forward
- Please correct answer and don't use hand ratingarrow_forwardConvert the following structures into a chair representation. Then conduct a chair flip. Cl a. b. C\.... оarrow_forwardAktiv Learning App Cengage Digital Learning Part of Speech Table for Assign x o Mail-Karen Ento-Outlook * + app.aktiv.com Your Aktiv Learning trial expires on 02/06/25 at 01:15 PM Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. Problem 17 of 30 Drawing Arrows heat 4 O M B D 5x H H Und Settings H Done :0: H Jararrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

Coenzymes and cofactors; Author: CH15 SWAYAM Prabha IIT Madras;https://www.youtube.com/watch?v=bubY2Nm7hVM;License: Standard YouTube License, CC-BY
Aromaticity and Huckel's Rule; Author: Professor Dave Explains;https://www.youtube.com/watch?v=7-BguH4_WBQ;License: Standard Youtube License