
Organic Chemistry (6th Edition)
6th Edition
ISBN: 9781260119107
Author: Janice Gorzynski Smith
Publisher: McGraw Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 7.13, Problem 25P
Classify each carbocation as
a. 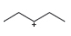 b.
b. 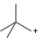 c.
c. 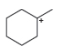 d.
d. 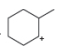
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Don't used Ai solution
3. Devise a retrosynthesis for the problem given below and then provide the corresponding
synthesis with all necessary reagents/reactants:
RETROSYNTHESIS:
SYNTHESIS:
Br
Several square planar complexes are known for Gold (III) ions but not for Silver (III) why?
Chapter 7 Solutions
Organic Chemistry (6th Edition)
Ch. 7.1 - Problem 7.1 Telfairine, a naturally occurring...Ch. 7.2 - Give the IUPAC name for each compound. a. b. c. d.Ch. 7.2 - Prob. 3PCh. 7.3 - An sp3 hybridized CCl bond is more polar than an...Ch. 7.4 - Prob. 5PCh. 7.6 - Prob. 6PCh. 7.7 - Prob. 10PCh. 7.8 - Prob. 15PCh. 7.8 - Prob. 16PCh. 7.8 - Prob. 17P
Ch. 7.11 - Prob. 18PCh. 7.11 - Prob. 19PCh. 7.11 - Draw the product of each SN2 reaction and indicate...Ch. 7.11 - Prob. 21PCh. 7.11 - Prob. 22PCh. 7.12 - What happens to the rate of an SN1 reaction under...Ch. 7.12 - Draw the products of each SN1 reaction and...Ch. 7.13 - Classify each carbocation as 1,2, or 3. a. b. c....Ch. 7.15 - Problem 7.30 For each alkyl halide and...Ch. 7.15 - Prob. 30PCh. 7.15 - Prob. 31PCh. 7.15 - Prob. 32PCh. 7.15 - Prob. 33PCh. 7.15 - Prob. 34PCh. 7 - Give the IUPAC name for each compound, including...Ch. 7 - Draw the products of each nucleophilic...Ch. 7 - Prob. 50PCh. 7 - Prob. 51PCh. 7 - 7.53 Consider the following reaction.
Draw a...Ch. 7 - Prob. 57PCh. 7 - Prob. 58PCh. 7 - Consider the following SN1 reaction. a.Draw a...Ch. 7 - 7.59 Pick the reactant or solvent in each part...Ch. 7 - Draw the products of each SN1 reaction and...Ch. 7 - Prob. 62PCh. 7 - Prob. 63PCh. 7 - Draw a stepwise, detailed mechanism for the...Ch. 7 - When a single compound contains both a nucleophile...Ch. 7 - Prob. 69PCh. 7 - Prob. 70PCh. 7 - Draw a stepwise, detailed mechanism f or the...Ch. 7 - Prob. 72PCh. 7 - Prob. 78P
Additional Science Textbook Solutions
Find more solutions based on key concepts
45. Calculate the mass of nitrogen dissolved at room temperature in an 80.0-L home aquarium. Assume a total pre...
Chemistry: Structure and Properties (2nd Edition)
Sea turtles have disappeared from many regions, and one way of trying to save them is to reintroduce them to ar...
MARINE BIOLOGY
Why is it unlikely that two neighboring water molecules would be arranged like this?
Campbell Biology (11th Edition)
Why do scientists think that all forms of life on earth have a common origin?
Genetics: From Genes to Genomes
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Aiter running various experiments, you determine that the mechanism for the following reaction is bimolecular. CI Using this information, draw the correct mechanism in the space below. X Explanation Check C Cl OH + CI Add/Remove step Click and drag to start drawing a structure. 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Carrow_forwardComplete the reaction in the fewest number of steps as possible, Draw all intermediates (In the same form as the picture provided) and provide all reagents.arrow_forwardPlease provide steps to work for complete understanding.arrow_forward
- Please provide steps to work for complete understanding.arrow_forwardIdentify the Functional Groups (FG) in the following molecules. Classify C atoms as tertiary, 30, or quaternary 40. Identify secondary 20 and tertiary, 30 hydrogen atoms. Please provide steps to undertand each labeling.arrow_forwardIdentify the Functional Groups (FG) in the following molecules. Classify C atoms as tertiary, 30, or quaternary 40. Identify secondary 20 and tertiary, 30 hydrogen atoms. Please provide steps to undertand each labeling.arrow_forward
- Identify the Functional Groups (FG) in the following molecules. Classify C atoms as tertiary, 30, or quaternary 40. Identify secondary 20 and tertiary, 30 hydrogen atoms. Please provide steps to undertand each labeling.arrow_forwardIdentify the Functional Groups (FG) in the following molecules. Classify C atoms as tertiary, 30, or quaternary 40. Identify secondary 20 and tertiary, 30 hydrogen atoms. Please provide steps to undertand each labeling.arrow_forwardA certain chemical reaction releases 24.7 kJ/g of heat for each gram of reactant consumed. How can you calculate what mass of reactant will produce 1460. J of heat? Set the math up. But don't do any of it. Just leave your answer as a math expression. Also, be sure your answer includes all the correct unit symbols. mass M 0.0 x μ 00 1 Garrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY