
CHEMISTRY-MASTERINGCHEMISTRY W/ETEXT
8th Edition
ISBN: 9780135204634
Author: Robinson
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 7, Problem 7.80SP
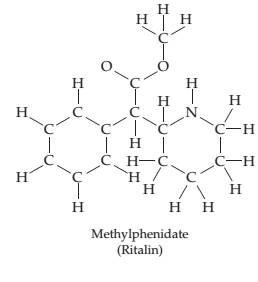
Methylphenidate
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
how many moles of H2O2 are required to react with 11g of N2H4 according to the following reaction? (atomic weights: N=14.01, H=1.008, O= 16.00) 7H2O2 + N2H4 -> 2HNO3 + 8H20
calculate the number of moles of H2 produced from 0.78 moles of Ga and 1.92 moles HCL? 2Ga+6HCL->2GaCl3+3H2
an adult human breathes 0.50L of air at 1 atm with each breath. If a 50L air tank at 200 atm is available, how man y breaths will the tank provide
Chapter 7 Solutions
CHEMISTRY-MASTERINGCHEMISTRY W/ETEXT
Ch. 7 - Use the electro negativity values in Figure 7.4...Ch. 7 - Conceptual APPLY 7.2 An electrostatic potential...Ch. 7 - The dipole moment of AgCI in the gas phaseis...Ch. 7 - Predict which bond has greater percent ionic...Ch. 7 - Select the correct electron-dot structure for H2S...Ch. 7 - Use the octet rule to predict the molecular...Ch. 7 - Identify the correct electron-dot structure for...Ch. 7 - Draw an electron-dot structure for each of the...Ch. 7 - Select the correct electron-dot structure for...Ch. 7 - Identify the correct electron-dot structure(s) for...
Ch. 7 - Prob. 7.11PCh. 7 - Which oxygen species do you predict to be most...Ch. 7 - Draw an electron-dot structure for the following...Ch. 7 - There are two molecules with the formula C2H6O...Ch. 7 - The following structure is a representation of...Ch. 7 - Draw two possible electron-dot structures for the...Ch. 7 - Called “laughing gas, nitrous oxide (N2O) is...Ch. 7 - Draw as many resonance structures as possible for...Ch. 7 - Prob. 7.19PCh. 7 - Prob. 7.20ACh. 7 - Calculate the formal charge on each atom in the...Ch. 7 - Start with the electron-dot structure for the...Ch. 7 - Calculate formal charges on the C and O atoms in...Ch. 7 - Three resonance structures for anisole (Problem...Ch. 7 - The toxicity of the organophosphate insecticides...Ch. 7 - Prob. 7.26PCh. 7 - Prob. 7.27PCh. 7 - The following structure is a representation of the...Ch. 7 - The electron-dot structure for the nerve a gentsar...Ch. 7 - Draw the new electron-dot structures indicated by...Ch. 7 - The following diagram shows the potential energy...Ch. 7 - The following diagram shows the potential energy...Ch. 7 - Two electrostatic potential maps are shown, one of...Ch. 7 - Prob. 7.34CPCh. 7 - Which of the following drawings is most likely to...Ch. 7 - The following ball-and-stick molecular model is a...Ch. 7 - The following hall-and-stick molecular model is a...Ch. 7 - Sinapaldehyde, a compound present in the toasted...Ch. 7 - Vitamin C (ascorbic acid) has the following...Ch. 7 - Match the following descriptions with the type of...Ch. 7 - Why do two atoms come together to form a covalent...Ch. 7 - Explain the difference in the bond dissociation...Ch. 7 - Explain the difference in the bond dissociation...Ch. 7 - Predict which of the following bonds should be...Ch. 7 - Prob. 7.45SPCh. 7 - What general trends in electro negativity occur in...Ch. 7 - Predict the electro negativity of the undiscovered...Ch. 7 - Order the following elements according to...Ch. 7 - Order the following elements according to...Ch. 7 - Which of the following substances contain bonds...Ch. 7 - Use the electro negativity data in Figure 7.4 to...Ch. 7 - Show the direction of polarity for each of the...Ch. 7 - Show the direction of polarity for each of the...Ch. 7 - Which of the substances...Ch. 7 - Which of the substances...Ch. 7 - Order the following compounds according to the...Ch. 7 - Order the following compounds according to the...Ch. 7 - Prob. 7.58SPCh. 7 - Using only the elements Ca, Cl, and Si, give...Ch. 7 - The dipole moment of BrCl is 0.518 D, and the...Ch. 7 - Prob. 7.61SPCh. 7 - Prob. 7.62SPCh. 7 - Prob. 7.63SPCh. 7 - Why does the octet rule apply primarily to...Ch. 7 - Which of the following substances contains an atom...Ch. 7 - Draw electron-dot structures for the following...Ch. 7 - Draw electron-dot structures for the following...Ch. 7 - Identify the correct electron-dot structure for...Ch. 7 - Draw an electron.dot structure for the hydronium...Ch. 7 - Oxalic acid, H2C2O4 , is a mildly poisonous...Ch. 7 - Draw an electron-dot structure for carbon...Ch. 7 - Prob. 7.72SPCh. 7 - Identify the fourth-row elements, X, that form the...Ch. 7 - Write electron-dot structures for molecules with...Ch. 7 - Write electron-dot structures for molecules with...Ch. 7 - Which compound do you expect to have the stronger...Ch. 7 - Which compound do you expect to have the stronger...Ch. 7 - Draw an electron-dot structure for each of the...Ch. 7 - Prob. 7.79SPCh. 7 - Methylphenidate (C14H19NO2) , marketed as Ritalin,...Ch. 7 - Pregabalin (C8H17NO2) , marketed as Lyric a, is an...Ch. 7 - The following molecular model is that of...Ch. 7 - Ibuprofen C 13 H 18 O 2 marketed under such brand...Ch. 7 - Draw as many resonance structures as you can that...Ch. 7 - Draw as many resonance structures as you can for...Ch. 7 - Which of the following pairs of structures...Ch. 7 - Which of the following pairs of structures...Ch. 7 - Draw as many resonance structures as you can that...Ch. 7 - Draw as many resonance structures as you can that...Ch. 7 - Benzene has the following structural formula. Use...Ch. 7 - Draw three resonance structures for sulfur...Ch. 7 - Some mothballs used when storing clothes are made...Ch. 7 - Four different structures (a), (b), (c), and (d)...Ch. 7 - Draw an electron-dot structure for carbon...Ch. 7 - Assign formal charges to the atoms in the...Ch. 7 - Assign formal charges to the atoms in the...Ch. 7 - Assign formal charges to the atoms in the...Ch. 7 - Assign formal charges to the atoms in the...Ch. 7 - Calculate formal charges for the C and O atoms in...Ch. 7 - Draw two electron-dot resonance structures that...Ch. 7 - Draw two electron-dot resonance structures that...Ch. 7 - Prob. 7.102SPCh. 7 - Prob. 7.103SPCh. 7 - Boron trifluoride reacts with dimethyl ether to...Ch. 7 - Thiofulminic acid, HCNS, has recently been...Ch. 7 - Draw two rcsonancc strutur for methyl isocyanate,...Ch. 7 - In the cyanatc ion. OCN , carbon is the central...Ch. 7 - Prob. 7.108MPCh. 7 - Prob. 7.109MPCh. 7 - Prob. 7.110MPCh. 7 - The neutral OH molecule has been implicated in...Ch. 7 - Prob. 7.112MPCh. 7 - Prob. 7.113MP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Using reaction free energy to predict equilibrium composition Consider the following equilibrium: 2NO2 (g) = N2O4(g) AGº = -5.4 kJ Now suppose a reaction vessel is filled with 4.53 atm of dinitrogen tetroxide (N2O4) at 279. °C. Answer the following questions about this system: Under these conditions, will the pressure of N2O4 tend to rise or fall? Is it possible to reverse this tendency by adding NO2? In other words, if you said the pressure of N2O4 will tend to rise, can that be changed to a tendency to fall by adding NO2? Similarly, if you said the pressure of N2O4 will tend to fall, can that be changed to a tendency to '2' rise by adding NO2? If you said the tendency can be reversed in the second question, calculate the minimum pressure of NO 2 needed to reverse it. Round your answer to 2 significant digits. 00 rise ☐ x10 fall yes no ☐ atm G Ar 1arrow_forwardWhy do we analyse salt?arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. H H CH3OH, H+ H Select to Add Arrows H° 0:0 'H + Q HH ■ Select to Add Arrows CH3OH, H* H. H CH3OH, H+ HH ■ Select to Add Arrows i Please select a drawing or reagent from the question areaarrow_forward
- What are examples of analytical methods that can be used to analyse salt in tomato sauce?arrow_forwardA common alkene starting material is shown below. Predict the major product for each reaction. Use a dash or wedge bond to indicate the relative stereochemistry of substituents on asymmetric centers, where applicable. Ignore any inorganic byproducts H Šali OH H OH Select to Edit Select to Draw 1. BH3-THF 1. Hg(OAc)2, H2O =U= 2. H2O2, NaOH 2. NaBH4, NaOH + Please select a drawing or reagent from the question areaarrow_forwardWhat is the MOHR titration & AOAC method? What is it and how does it work? How can it be used to quantify salt in a sample?arrow_forward
- Predict the major products of this reaction. Cl₂ hv ? Draw only the major product or products in the drawing area below. If there's more than one major product, you can draw them in any arrangement you like. Be sure you use wedge and dash bonds if necessary, for example to distinguish between major products with different stereochemistry. If there will be no products because there will be no significant reaction, just check the box under the drawing area and leave it blank. Note for advanced students: you can ignore any products of repeated addition. Explanation Check Click and drag to start drawing a structure. 80 10 m 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility DII A F1 F2 F3 F4 F5 F6 F7 F8 EO F11arrow_forwardGiven a system with an anodic overpotential, the variation of η as a function of current density- at low fields is linear.- at higher fields, it follows Tafel's law.Calculate the range of current densities for which the overpotential has the same value when calculated for both cases (the maximum relative difference will be 5%, compared to the behavior for higher fields).arrow_forwardUsing reaction free energy to predict equilibrium composition Consider the following equilibrium: N2 (g) + 3H2 (g) = 2NH3 (g) AGº = -34. KJ Now suppose a reaction vessel is filled with 8.06 atm of nitrogen (N2) and 2.58 atm of ammonia (NH3) at 106. °C. Answer the following questions about this system: rise Under these conditions, will the pressure of N2 tend to rise or fall? ☐ x10 fall Is it possible to reverse this tendency by adding H₂? In other words, if you said the pressure of N2 will tend to rise, can that be changed to a tendency to fall by adding H2? Similarly, if you said the pressure of N will tend to fall, can that be changed to a tendency to rise by adding H₂? If you said the tendency can be reversed in the second question, calculate the minimum pressure of H₂ needed to reverse it. Round your answer to 2 significant digits. yes no ☐ atm Х ด ? olo 18 Ararrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY