
(a)
Interpretation:
The curved arrow notations showing all possible
Concept introduction:
Curved arrows are used to represent the movement of electrons in a reaction mechanism. The arrow starts on an electron-rich atom or an electron-rich region such as a pi bond. It ends on an electron poor atom when the movement results in the formation of a new sigma bond. If the result is the formation of a pi bond, the arrow ends in the region between the two atoms that form the bond.
A carbocation is a positively charged carbon atom that is electron-poor, two electrons short of an octet. It is unstable because it is a charged species.
A
A
The numbering in the shift label simply signifies that a hydride or a methyl group migrates from one carbon to an adjacent one. The numbering is not related to the root chain atom numbering.
Answer to Problem 7.36P
The possible
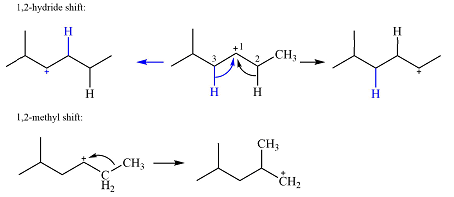
Explanation of Solution
The structure of the carbocation is
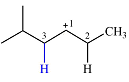
The charge is on the carbon numbered 1. There are two hydrogen atoms on the adjacent carbons C2 and C3. These are the ones that can shift to C1 in the two possible
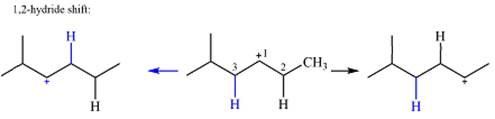
There is only one methyl group on the carbon adjacent to C1, attached to C2. Shifting of this methyl to C1 results in shifting of the charge to C2. Therefore, the
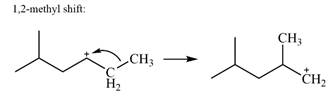
A hydride (
(b)
Interpretation:
The curved arrow notations showing all possible
Concept introduction:
Curved arrows are used to represent the movement of electrons in a reaction mechanism. The arrow starts on an electron-rich atom or an electron-rich region such as a pi bond. It ends on an electron poor atom when the movement results in the formation of a new sigma bond. If the result is the formation of a pi bond, the arrow ends in the region between the two atoms that form the bond.
A carbocation is a positively charged carbon atom that is electron-poor, two electrons short of an octet. It is unstable because it is a charged species.
A
A
The numbering in the shift label simply signifies that a hydride or a methyl group migrates from one carbon to an adjacent one. The numbering is not related to the root chain atom numbering.
Answer to Problem 7.36P
The possible
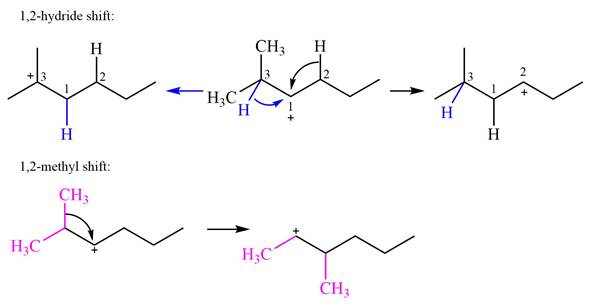
Explanation of Solution
The structure of the given carbocation is
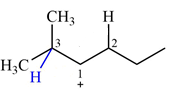
There are two hydrogen atoms on adjacent carbons C2 and C3 that can shift to the positively charged carbon C1 in two possible
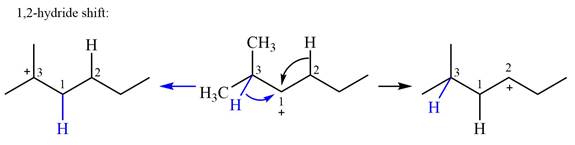
There are two methyl groups attached to a carbon adjacent to C1. Both are on the same carbon C3, therefore, shifting of either one will give the same product.
Therefore, the
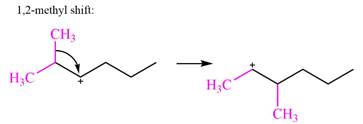
A hydride (
(c)
Interpretation:
The curved arrow notations showing all possible
Concept introduction:
Curved arrows are used to represent the movement of electrons in a reaction mechanism. The arrow starts on an electron-rich atom or an electron-rich region such as a pi bond. It ends on an electron poor atom when the movement results in the formation of a new sigma bond. If the result is the formation of a pi bond, the arrow ends in the region between the two atoms that form the bond.
A carbocation is a positively charged carbon atom that is electron-poor, two electrons short of an octet. It is unstable because it is a charged species.
A
A
The numbering in the shift label simply signifies that a hydride or a methyl group migrates from one carbon to an adjacent one. The numbering is not related to the root chain atom numbering.
Answer to Problem 7.36P
The possible
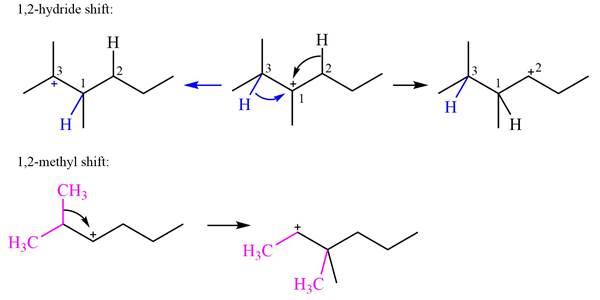
Explanation of Solution
The structure of the given carbocation is
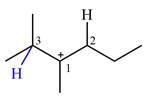
There are two hydrogen atoms on adjacent carbons C2 and C3 that can shift to the positively charged carbon C1 in two possible
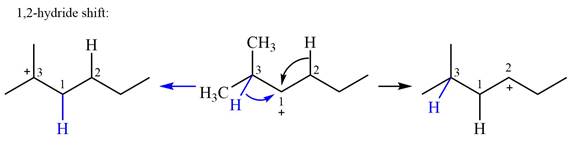
There are two methyl groups attached to a carbon adjacent to C1. Both are on the same carbon C3; therefore, shifting of either one will give the same product.
Therefore, the
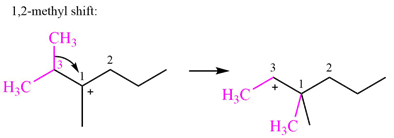
A hydride (
(d)
Interpretation:
The curved arrow notations showing all possible
Concept introduction:
Curved arrows are used to represent the movement of electrons in a reaction mechanism. The arrow starts on an electron-rich atom or an electron-rich region such as a pi bond. It ends on an electron poor atom when the movement results in the formation of a new sigma bond. If the result is the formation of a pi bond, the arrow ends in the region between the two atoms that form the bond.
A carbocation is a positively charged carbon atom that is electron-poor, two electrons short of an octet. It is unstable because it is a charged species.
A
A
The numbering in the shift label simply signifies that a hydride or a methyl group migrates from one carbon to an adjacent one. The numbering is not related to the root chain atom numbering.
Answer to Problem 7.36P
The possible
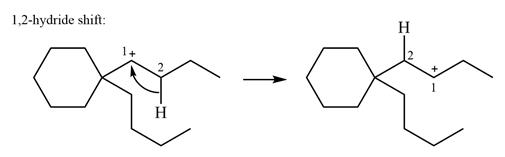
As there are no methyl groups on the carbon adjacent to the charge bearing carbon C1, a
Explanation of Solution
The structure of the given carbocation is
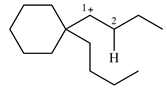
There is only one hydrogen atom on an adjacent carbon, C2, that can shift to the positively charged carbon C1 in a possible
Therefore, the
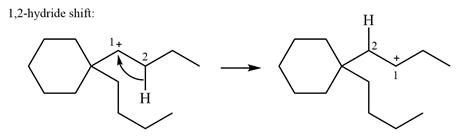
There are no methyl groups attached to the carbon adjacent to C1. Therefore, a
A hydride (
(e)
Interpretation:
The curved arrow notations showing all possible
Concept introduction:
Curved arrows are used to represent the movement of electrons in a reaction mechanism. The arrow starts on an electron-rich atom or an electron-rich region such as a pi bond. It ends on an electron poor atom when the movement results in the formation of a new sigma bond. If the result is the formation of a pi bond, the arrow ends in the region between the two atoms that form the bond.
A carbocation is a positively charged carbon atom that is electron-poor, two electrons short of an octet. It is unstable because it is a charged species.
A
A
The numbering in the shift label simply signifies that a hydride or a methyl group migrates from one carbon to an adjacent one. The numbering is not related to the root chain atom numbering.
Answer to Problem 7.36P
The possible
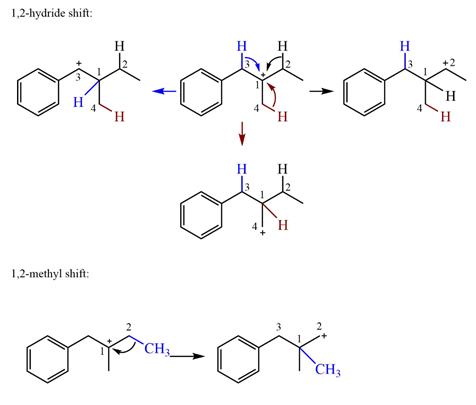
Explanation of Solution
The structure of the given carbocation is
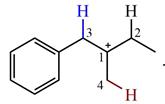
There are three hydrogen atoms on carbon atoms adjacent to the charge carrying carbon. They are on C2, C3, and C4.
Shifting of the hydride on C2 results in the charge shifting to C2, as shown in the product on the right.
Shifting of the hydride on C3 results in the charge shifting to C3, as shown in the product on the left.
Shifting of the hydride on C4 results in the charge shifting to C4, as shown in the product below the given carbocation.
Therefore, the
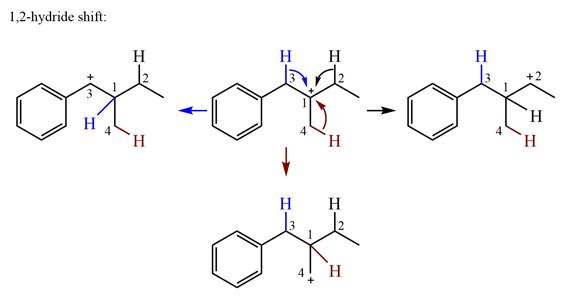
There is one methyl group attached to the carbon adjacent to C1. Shifting of the methyl group on C2 to C1 results in C1 becoming a tertiary carbon and the charge shifting to C2.
Therefore, the possible
A hydride (
(f)
Interpretation:
The curved arrow notations showing all possible
Concept introduction:
Curved arrows are used to represent the movement of electrons in a reaction mechanism. The arrow starts on an electron-rich atom or an electron-rich region such as a pi bond. It ends on an electron poor atom when the movement results in the formation of a new sigma bond. If the result is the formation of a pi bond, the arrow ends in the region between the two atoms that form the bond.
A carbocation is a positively charged carbon atom that is electron-poor, two electrons short of an octet. It is unstable because it is a charged species.
A
A
The numbering in the shift label simply signifies that a hydride or a methyl group migrates from one carbon to an adjacent one. The numbering is not related to the root chain atom numbering.
Answer to Problem 7.36P
The possible
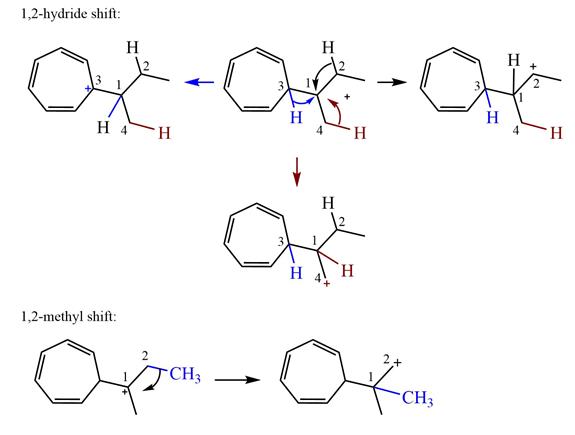
Explanation of Solution
The structure of the given carbocation is
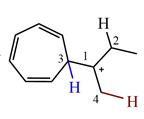
There are three hydrogen atoms on carbon atoms adjacent to the charge carrying carbon. They are on C2, C3, and C4.
Shifting of the hydride on C2 results in the charge shifting to C2, as shown in the product on the right.
Shifting of the hydride on C3 results in the charge shifting to C3, as shown in the product on the left.
Shifting of the hydride on C4 results in the charge shifting to C4, as shown in the product below the given carbocation.
Therefore, the
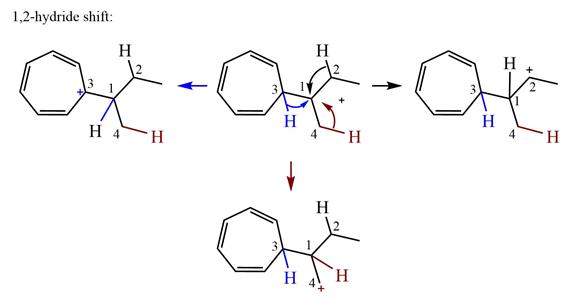
There is one methyl group attached to a carbon adjacent to C1. Shifting of the methyl group on C2 to C1 results in C1 becoming a tertiary carbon and the charge shifting to C2.
Therefore, the possible
A hydride (
Want to see more full solutions like this?
Chapter 7 Solutions
EBK ORGANIC CHEMISTRY: PRINCIPLES AND M
- The vapor pressure of dichloromethane at 20.0 °C is 58.0 kPa and its enthalpy of vaporization is 32.7 kJ/mol. Estimate the temperature at which its vapor pressure is 66.0 kPa.arrow_forwardDraw the structure of A, the minor E1 product of the reaction. Cl Skip Part Check F1 esc CH_CH OH, D 3 2 Click and drag to start drawing a structure. 80 R3 F4 F2 F3 @ 2 # $ 4 3 Q W 95 % KO 5 F6 A F7 × G ☐ Save For Later Sub 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy C ►II A A F8 F9 F10 FL 6 7 88 & * 8 9 LLI E R T Y U A S D lock LL F G H 0 P J K L Z X C V B N M 9 Harrow_forwardFrom the choices given, which two substances have the same crystal structure? (Select both) Group of answer choices ZnS (zincblende) Diamond TiO2 (rutile) ZnS (wurtzite)arrow_forward
- Potassium (K) blends with germanium (Ge) to form a Zintl phase with a chemical formula of K4Ge4. Which of the following elements would you expect potassium to blend with to form an alloy? Electronegativities: As (2.0), Cl (3.0), Ge (1.8), K (0.8), S (2.5), Ti (1.5) Group of answer choices Arsenic (As) Sulfur (S) Chlorine (Cl) Titanium (Ti)arrow_forwardConsider two elements, X and Z. Both have cubic-based unit cells with the same edge lengths. X has a bcc unit cell while Z has a fcc unit cell. Which of the following statements is TRUE? Group of answer choices Z has a larger density than X X has more particles in its unit cell than Z does X has a larger density than Z Z has a larger unit cell volume than Xarrow_forwardHow many particles does a face-centered cubic (fcc) unit cell contain? Group of answer choices 2 14 8 4arrow_forward
- V Highlight all of the carbon atoms that have at least one beta (B) hydrogen, using red for one ẞ hydrogen, blue for two ẞ hydrogens, and green for three ẞ hydrogens. If none of the carbon atoms have ẞ hydrogens, check the box underneath the molecule. ED X None of the carbon atoms have ẞ hydrogens. Explanation esc 2 Check * F1 F2 1 2 80 # 3 Q W tab A caps lock shift fn control F3 N S option O 694 $ F4 F5 F6 005 % E R D F LL 6 olo 18 Ar B © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility A DII F7 F8 87 & * 8 T Y U G H 4 F9 F10 ( 9 0 E F11 F12 உ J K L + || X C V B N M H H command option commandarrow_forwardConsider the reaction below and answer the following questions. Part 1 of 4 Br NaOCH2CH3 Identify the mechanisms involved. Check all that apply. SN 1 SN 2 E1 E2 None of the above Part 2 of 4 Skip Part Check esc F1 F2 lock 1 2 Q W A S #3 80 F3 F4 F5 F6 Save For © 2025 McGraw Hill LLC. All Rights Reserved. Terms ˇˇ % & 4 5 6 89 7 IK A 分 བ F7 F8 F9 F * E R T Y U 8 9 D F G H K V B N M 0 Oarrow_forwardWhat kind of holes are not generated when solid-state particles adopt a close packing pattern? Group of answer choices tetrahedral cubic octahedral None of the other choices are correctarrow_forward
- For the reaction below: 1. Draw all reasonable elimination products to the right of the arrow. 2. In the box below the reaction, redraw any product you expect to be a major product. 田 Major Product: Check ☐ + I Na OH esc F1 F2 2 1 @ 2 Q W tab A caps lock S #3 80 F3 69 4 σ F4 % 95 S Click and drag to sta drawing a structure mm Save For Later 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use GO DII F5 F6 F7 F8 F9 F10 6 CO 89 & 7 LU E R T Y U 8* 9 0 D F G H J K L Z X C V B N M 36arrow_forwardProblem 7 of 10 Draw the major product of this reaction. Ignore inorganic byproducts. S' S 1. BuLi 2. ethylene oxide (C2H4O) Select to Draw a Submitarrow_forwardFeedback (4/10) 30% Retry Curved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the arrows to draw the reactant and missing intermediates involved in this reaction. Include all lone pairs and charges as appropriate. Ignore inorganic byproducts. Incorrect, 6 attempts remaining :0: Draw the Reactant H H3CO H- HIO: Ö-CH3 CH3OH2* protonation H. a H (+) H Ο CH3OH2 O: H3C protonation CH3OH deprotonation > CH3OH nucleophilic addition H. HO 0:0 Draw Intermediate a Xarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
