
Concept explainers
(a)
Interpretation:
Whether the given substrate undergoes E2 elimination step with base
Concept introduction:
Answer to Problem 7.29P
The given substrate cannot undergo E2 elimination step with base
Explanation of Solution
The given substrate is
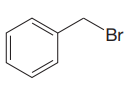
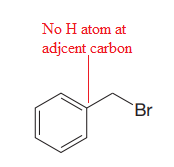
Thus, the given substrate cannot undergo
No H atom on adjacent carbon atom.
(b)
Interpretation:
Whether the given substrate undergoes E2 elimination step with base
Concept introduction:
Answer to Problem 7.29P
The given substrate undergoes E2 elimination step.
The E2 elimination step for the given substrate is drawn as:
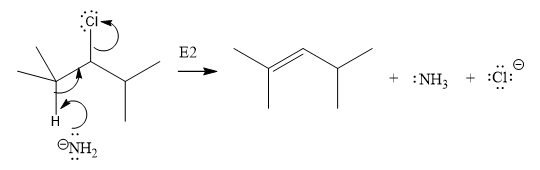
Explanation of Solution
The given substrate is:
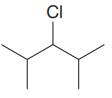
In the above given substrate, Cl acts as a leaving group with one H atom on each side. Any one of the hydrogen atom is taken away forming a C=C bond. The first curved arrow is drawn from the lone pair of nitrogen atom of a base

The product of
(c)
Interpretation:
Whether the given substrate undergo E2 elimination step with base
Concept introduction:
Answer to Problem 7.29P
The given substrate undergoes E2 elimination step.
The E2 elimination step for the given substrate is drawn as:
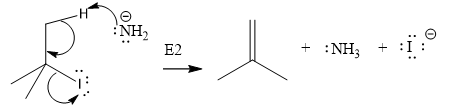
Explanation of Solution
The given substrate is:
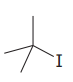
In the above given substrate,
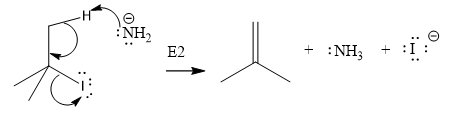
The product of
(d)
Interpretation:
Whether the given substrate undergoes E2 elimination step with base
Concept introduction:
Answer to Problem 7.29P
The given substrate cannot undergoes E2 elimination step with base
Explanation of Solution
The given substrate is
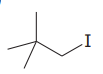
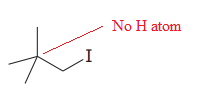
Thus, the given substrate cannot undergo
There is no H atom on the adjacent carbon atom.
(e)
Interpretation:
Whether the given substrate undergo E2 elimination step with base
Concept introduction:
The
Answer to Problem 7.29P
The given substrate undergoes E2 elimination step.
The E2 elimination step for the given substrate is drawn as:
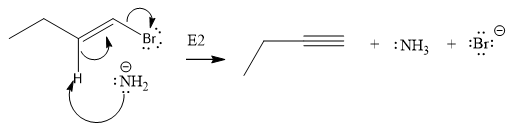
Explanation of Solution
The given substrate is
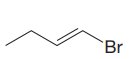
In the above given substrate,
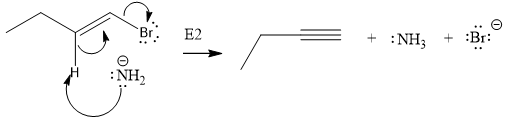
The product of
Want to see more full solutions like this?
Chapter 7 Solutions
EBK ORGANIC CHEMISTRY: PRINCIPLES AND M
- Part I. Draw reaction mechanism for the transformations of benzophenone to benzopinacol to benzopinaco lone and answer the ff: Pinacol (2,3-dimethyl, 1-3-butanediol) on treatment w/ acid gives a mixture of pina colone and (3,3-dimethyl-2-butanone) 2,3-dimethyl-1,3-butadiene. Give reasonable mechanism the formation of the products Forarrow_forwardShow the mechanism for these reactionsarrow_forwardDraw the stepwise mechanismarrow_forward
- Draw a structural formula of the principal product formed when benzonitrile is treated with each reagent. (a) H₂O (one equivalent), H₂SO₄, heat (b) H₂O (excess), H₂SO₄, heat (c) NaOH, H₂O, heat (d) LiAlH4, then H₂Oarrow_forwardDraw the stepwise mechanism for the reactionsarrow_forwardDraw stepwise mechanismarrow_forward
- Part I. Draw reaction mechanism for the transformations of benzophenone to benzopinacol to benzopinaco lone and answer the ff: a) Give the major reason for the exposure of benzophenone al isopropyl alcohol (w/acid) to direct sunlight of pina colone Mechanism For b) Pinacol (2,3-dimethy 1, 1-3-butanediol) on treatment w/ acid gives a mixture (3,3-dimethyl-2-butanone) and 2, 3-dimethyl-1,3-butadiene. Give reasonable the formation of the productsarrow_forwardwhat are the Iupac names for each structurearrow_forwardWhat are the IUPAC Names of all the compounds in the picture?arrow_forward
- 1) a) Give the dominant Intermolecular Force (IMF) in a sample of each of the following compounds. Please show your work. (8) SF2, CH,OH, C₂H₂ b) Based on your answers given above, list the compounds in order of their Boiling Point from low to high. (8)arrow_forward19.78 Write the products of the following sequences of reactions. Refer to your reaction road- maps to see how the combined reactions allow you to "navigate" between the different functional groups. Note that you will need your old Chapters 6-11 and Chapters 15-18 roadmaps along with your new Chapter 19 roadmap for these. (a) 1. BHS 2. H₂O₂ 3. H₂CrO4 4. SOCI₂ (b) 1. Cl₂/hv 2. KOLBU 3. H₂O, catalytic H₂SO4 4. H₂CrO4 Reaction Roadmap An alkene 5. EtOH 6.0.5 Equiv. NaOEt/EtOH 7. Mild H₂O An alkane 1.0 2. (CH3)₂S 3. H₂CrO (d) (c) 4. Excess EtOH, catalytic H₂SO OH 4. Mild H₂O* 5.0.5 Equiv. NaOEt/EtOH An alkene 6. Mild H₂O* A carboxylic acid 7. Mild H₂O* 1. SOC₁₂ 2. EtOH 3.0.5 Equiv. NaOEt/E:OH 5.1.0 Equiv. NaOEt 6. NH₂ (e) 1. 0.5 Equiv. NaOEt/EtOH 2. Mild H₂O* Br (f) i H An aldehyde 1. Catalytic NaOE/EtOH 2. H₂O*, heat 3. (CH,CH₂)₂Culi 4. Mild H₂O* 5.1.0 Equiv. LDA Br An ester 4. NaOH, H₂O 5. Mild H₂O* 6. Heat 7. MgBr 8. Mild H₂O* 7. Mild H₂O+arrow_forwardLi+ is a hard acid. With this in mind, which if the following compounds should be most soluble in water? Group of answer choices LiBr LiI LiF LiClarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
