
LCPO CHEMISTRY W/MODIFIED MASTERING
8th Edition
ISBN: 9780135214756
Author: Robinson
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 7, Problem 7.24A
Three resonance structures for anisole (Problem 7.20) are shown. Calculate formal charges on C and O atoms and decide which structure makes the largest contribution to the resonance hybrid.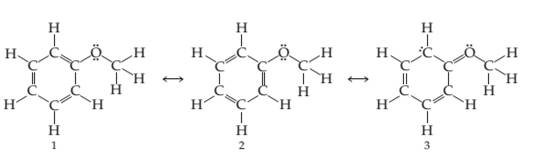
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
in the kinetics experiment, what were the values calculated? Select all that apply.a) equilibrium constantb) pHc) order of reactiond) rate contstant
true or false, given that a 20.00 mL sample of NaOH took 24.15 mL of 0.141 M HCI to reach the endpoint in a titration, the concentration of the NaOH is 1.17 M.
in the bromothymol blue experiment, pKa was measured. A closely related compound has a Ka of 2.10 x 10-5. What is the pKa?a) 7.1b) 4.7c) 2.0
Chapter 7 Solutions
LCPO CHEMISTRY W/MODIFIED MASTERING
Ch. 7 - Use the electro negativity values in Figure 7.4...Ch. 7 - Conceptual APPLY 7.2 An electrostatic potential...Ch. 7 - The dipole moment of AgCI in the gas phaseis...Ch. 7 - Predict which bond has greater percent ionic...Ch. 7 - Select the correct electron-dot structure for H2S...Ch. 7 - Use the octet rule to predict the molecular...Ch. 7 - Identify the correct electron-dot structure for...Ch. 7 - Draw an electron-dot structure for each of the...Ch. 7 - Select the correct electron-dot structure for...Ch. 7 - Identify the correct electron-dot structure(s) for...
Ch. 7 - Prob. 7.11PCh. 7 - Which oxygen species do you predict to be most...Ch. 7 - Draw an electron-dot structure for the following...Ch. 7 - There are two molecules with the formula C2H6O...Ch. 7 - The following structure is a representation of...Ch. 7 - Draw two possible electron-dot structures for the...Ch. 7 - Called “laughing gas, nitrous oxide (N2O) is...Ch. 7 - Draw as many resonance structures as possible for...Ch. 7 - Prob. 7.19PCh. 7 - Prob. 7.20ACh. 7 - Calculate the formal charge on each atom in the...Ch. 7 - Start with the electron-dot structure for the...Ch. 7 - Calculate formal charges on the C and O atoms in...Ch. 7 - Three resonance structures for anisole (Problem...Ch. 7 - The toxicity of the organophosphate insecticides...Ch. 7 - Prob. 7.26PCh. 7 - Prob. 7.27PCh. 7 - The following structure is a representation of the...Ch. 7 - The electron-dot structure for the nerve a gentsar...Ch. 7 - Draw the new electron-dot structures indicated by...Ch. 7 - The following diagram shows the potential energy...Ch. 7 - The following diagram shows the potential energy...Ch. 7 - Two electrostatic potential maps are shown, one of...Ch. 7 - Prob. 7.34CPCh. 7 - Which of the following drawings is most likely to...Ch. 7 - The following ball-and-stick molecular model is a...Ch. 7 - The following hall-and-stick molecular model is a...Ch. 7 - Sinapaldehyde, a compound present in the toasted...Ch. 7 - Vitamin C (ascorbic acid) has the following...Ch. 7 - Match the following descriptions with the type of...Ch. 7 - Why do two atoms come together to form a covalent...Ch. 7 - Explain the difference in the bond dissociation...Ch. 7 - Explain the difference in the bond dissociation...Ch. 7 - Predict which of the following bonds should be...Ch. 7 - Prob. 7.45SPCh. 7 - What general trends in electro negativity occur in...Ch. 7 - Predict the electro negativity of the undiscovered...Ch. 7 - Order the following elements according to...Ch. 7 - Order the following elements according to...Ch. 7 - Which of the following substances contain bonds...Ch. 7 - Use the electro negativity data in Figure 7.4 to...Ch. 7 - Show the direction of polarity for each of the...Ch. 7 - Show the direction of polarity for each of the...Ch. 7 - Which of the substances...Ch. 7 - Which of the substances...Ch. 7 - Order the following compounds according to the...Ch. 7 - Order the following compounds according to the...Ch. 7 - Prob. 7.58SPCh. 7 - Using only the elements Ca, Cl, and Si, give...Ch. 7 - The dipole moment of BrCl is 0.518 D, and the...Ch. 7 - Prob. 7.61SPCh. 7 - Prob. 7.62SPCh. 7 - Prob. 7.63SPCh. 7 - Why does the octet rule apply primarily to...Ch. 7 - Which of the following substances contains an atom...Ch. 7 - Draw electron-dot structures for the following...Ch. 7 - Draw electron-dot structures for the following...Ch. 7 - Identify the correct electron-dot structure for...Ch. 7 - Draw an electron.dot structure for the hydronium...Ch. 7 - Oxalic acid, H2C2O4 , is a mildly poisonous...Ch. 7 - Draw an electron-dot structure for carbon...Ch. 7 - Prob. 7.72SPCh. 7 - Identify the fourth-row elements, X, that form the...Ch. 7 - Write electron-dot structures for molecules with...Ch. 7 - Write electron-dot structures for molecules with...Ch. 7 - Which compound do you expect to have the stronger...Ch. 7 - Which compound do you expect to have the stronger...Ch. 7 - Draw an electron-dot structure for each of the...Ch. 7 - Prob. 7.79SPCh. 7 - Methylphenidate (C14H19NO2) , marketed as Ritalin,...Ch. 7 - Pregabalin (C8H17NO2) , marketed as Lyric a, is an...Ch. 7 - The following molecular model is that of...Ch. 7 - Ibuprofen C 13 H 18 O 2 marketed under such brand...Ch. 7 - Draw as many resonance structures as you can that...Ch. 7 - Draw as many resonance structures as you can for...Ch. 7 - Which of the following pairs of structures...Ch. 7 - Which of the following pairs of structures...Ch. 7 - Draw as many resonance structures as you can that...Ch. 7 - Draw as many resonance structures as you can that...Ch. 7 - Benzene has the following structural formula. Use...Ch. 7 - Draw three resonance structures for sulfur...Ch. 7 - Some mothballs used when storing clothes are made...Ch. 7 - Four different structures (a), (b), (c), and (d)...Ch. 7 - Draw an electron-dot structure for carbon...Ch. 7 - Assign formal charges to the atoms in the...Ch. 7 - Assign formal charges to the atoms in the...Ch. 7 - Assign formal charges to the atoms in the...Ch. 7 - Assign formal charges to the atoms in the...Ch. 7 - Calculate formal charges for the C and O atoms in...Ch. 7 - Draw two electron-dot resonance structures that...Ch. 7 - Draw two electron-dot resonance structures that...Ch. 7 - Prob. 7.102SPCh. 7 - Prob. 7.103SPCh. 7 - Boron trifluoride reacts with dimethyl ether to...Ch. 7 - Thiofulminic acid, HCNS, has recently been...Ch. 7 - Draw two rcsonancc strutur for methyl isocyanate,...Ch. 7 - In the cyanatc ion. OCN , carbon is the central...Ch. 7 - Prob. 7.108MPCh. 7 - Prob. 7.109MPCh. 7 - Prob. 7.110MPCh. 7 - The neutral OH molecule has been implicated in...Ch. 7 - Prob. 7.112MPCh. 7 - Prob. 7.113MP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- calculate the equilibrium concentration of H2 given that K= 0.017 at a constant temperature for this reaction. The inital concentration of HBr is 0.050 M.2HBr(g) ↔ H2(g) + Br2(g)a) 4.48 x 10-2 M b) 5.17 x 10-3 Mc) 1.03 x 10-2 Md) 1.70 x 10-2 Marrow_forwardtrue or falsegiven these two equilibria with their equilibrium constants:H2(g) + CI2(l) ↔ 2HCI(g) K= 0.006 CI2(l) ↔ CI2(g) K= 0.30The equilibrium contstant for the following reaction is 1.8H2(g) + CI2 ↔ 2HCI(g)arrow_forwardI2(g) + CI2(g) ↔ 2ICIK for this reaction is 81.9. Find the equilibrium concentration of I2 if the inital concentration of I2 and CI2 are 0.010 Marrow_forward
- true or false,the equilibrium constant for this reaction is 0.50.PCI5(g) ↔ PCI3(g) + CI2(g)Based on the above, the equilibrium constant for the following reaction is 0.25.2PCI5(g) ↔. 2PCI3(g) + 2CI2(g)arrow_forwardtrue or false, using the following equilibrium, if carbon dioxide is added the equilibrium will shift toward the productsC(s) + CO2(g) ↔ 2CO(g)arrow_forward2S2O2/3- (aq) + I2 (aq) ---> S4O2/6- (aq) +2I- (aq) Experiment I2 (M) S2O3- (M) Initital Rate (M/s) 1 0.01 0.01 0.0004 2 0.01 0.02 0.0004 3 0.02 0.01 0.0008 Calculate the overall order for this reaction using the table data a) 3b) 0c) 2d) 1arrow_forward
- the decomposition of N2O5 is the first order with a half-life of 1.98 minutes. If the inital concentration of N2O5 is 0.200 M, what is the concentration after 6 minutes?a) 0.612 Mb) 0.035 Mc) 0.024 Md) 0.100 Marrow_forward20.00 mL of 0.150 M HCI is titrated with 0.075 M NaOH. What volume of NaOH is needed?a) 50 mLb) 20 mLc) 40 mLd) 26.66 mLarrow_forward20.00 mL of 0.150 M NaOH is titrated with 37.75 mL of HCI. What is the molarity of the HCI?a) 0.150 Mb) 0.079 Mc) 0.025 Md) 0.050 Marrow_forward
- in the following reaction, the OH- acts as which of these?NO2- (aq) + H2O (l) ⇌ OH- (aq) + HNO2 (aq)a) not a weak acidb) basec) acidarrow_forwardfind the pH of a buffer made from 0.20 M HNO2 and 0.10 M NaNO2. Ka= 4.0 x 10-4a) 4.00b) 3.40c) 3.70d) 3.10arrow_forwardthe Ka for sodium dihydrogen phosphate is 6.32 x 10-8. Find the pH of a buffer made from 0.15 M H2PO4- and 0.15 M HPO42-.a) 6.98b) 7.42c) 7.00d) 7.20arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY