
Interpretation:
The polar molecules has to be found from the given set of options.
Concept Introduction:
Polarity is a term that is used to explain the separation of electric charge in a molecule. A molecule is said to be polar if it contains atoms of different electronegativity bonded together. Dipole moment is the measure of polarity of the molecule. Dipole moment is the product of distance between the charges and the magnitude of electric charge, it is a vector quantity. Even though a molecule is having atoms of different electronegativity bonded together if the geometry of the molecule is symmetric, then the resultant dipole moment cancels each other and the molecule becomes non-polar.
Water is an example of polar molecule. From VSEPR theory the shape of the water is found to be bent. The direction of dipole moment is given in the Figure 1. Since the structure is not symmetrical the dipole is not cancelling each other.
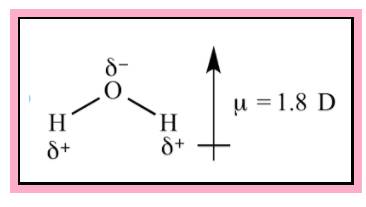
Figure 1
The linear carbon dioxide molecule is an example of non-polar molecule that is having polar bonds. Because of the symmetric structure the dipole moment cancels each other.
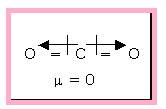
Figure 2
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
Chemistry: Atoms First V1
- Including activity, calculate the solubility of Pb(IO3)2 in a matrix of 0.020 M Mg(NO3)2.arrow_forwardIncluding activity coefficients, find [Hg22+] in saturated Hg2Br2 in 0.00100 M KBr.arrow_forwardIncluding activity, calculate the pH of a 0.010 M HCl solution with an ionic strength of 0.10 M.arrow_forward
- Can I please get the graph 1: Concentration vs. Density?arrow_forwardOrder the following series of compounds from highest to lowest reactivity to electrophilic aromatic substitution, explaining your answer: 2-nitrophenol, p-Toluidine, N-(4-methylphenyl)acetamide, 4-methylbenzonitrile, 4-(trifluoromethyl)benzonitrile.arrow_forwardOrdene la siguiente serie de compuestos de mayor a menor reactividad a la sustitución aromática electrofílica, explicando su respuesta: ácido bencenosulfónico, fluorobenceno, etilbenceno, clorobenceno, terc-butilbenceno, acetofenona.arrow_forward
- Can I please get all final concentrations please!arrow_forwardState the detailed mechanism of the reaction of benzene with isopropanol in sulfuric acid.arrow_forwardDo not apply the calculations, based on the approximation of the stationary state, to make them perform correctly. Basta discard the 3 responses that you encounter that are obviously erroneous if you apply the formula to determine the speed of a reaction. For the decomposition reaction of N2O5(g): 2 N2O5(g) · 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 -> NO2 + NO3_(K1) NO2 + NO3 →> N2O5 (k-1) → NO2 + NO3 → NO2 + O2 + NO (K2) NO + N2O5 → NO2 + NO2 + NO2 (K3) Give the expression for the acceptable rate. (A). d[N₂O] dt = -1 2k,k₂[N205] k₁+k₂ d[N₂O5] (B). dt =-k₁[N₂O₂] + k₁[NO2][NO3] - k₂[NO2]³ (C). d[N₂O] dt =-k₁[N₂O] + k₁[N205] - K3 [NO] [N205] (D). d[N2O5] =-k₁[NO] - K3[NO] [N₂05] dtarrow_forward
- A 0.10 M solution of acetic acid (CH3COOH, Ka = 1.8 x 10^-5) is titrated with a 0.0250 M solution of magnesium hydroxide (Mg(OH)2). If 10.0 mL of the acid solution is titrated with 20.0 mL of the base solution, what is the pH of the resulting solution?arrow_forwardFor the decomposition reaction of N2O5(g): 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 NO2 + NO3 (K1) | NO2 + NO3 → N2O5 (k-1) | NO2 + NO3 NO2 + O2 + NO (k2) | NO + N2O51 NO2 + NO2 + NO2 (K3) → Give the expression for the acceptable rate. → → (A). d[N205] dt == 2k,k₂[N₂O₂] k₁+k₁₂ (B). d[N2O5] =-k₁[N₂O] + k₁[NO₂] [NO3] - k₂[NO₂]³ dt (C). d[N2O5] =-k₁[N₂O] + k [NO] - k₂[NO] [NO] d[N2O5] (D). = dt = -k₁[N2O5] - k¸[NO][N₂05] dt Do not apply the calculations, based on the approximation of the stationary state, to make them perform correctly. Basta discard the 3 responses that you encounter that are obviously erroneous if you apply the formula to determine the speed of a reaction.arrow_forwardFor the decomposition reaction of N2O5(g): 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 NO2 + NO3 (K1) | NO2 + NO3 → N2O5 (k-1) | NO2 + NO3 NO2 + O2 + NO (k2) | NO + N2O51 NO2 + NO2 + NO2 (K3) → Give the expression for the acceptable rate. → → (A). d[N205] dt == 2k,k₂[N₂O₂] k₁+k₁₂ (B). d[N2O5] =-k₁[N₂O] + k₁[NO₂] [NO3] - k₂[NO₂]³ dt (C). d[N2O5] =-k₁[N₂O] + k [NO] - k₂[NO] [NO] d[N2O5] (D). = dt = -k₁[N2O5] - k¸[NO][N₂05] dt Do not apply the calculations, based on the approximation of the stationary state, to make them perform correctly. Basta discard the 3 responses that you encounter that are obviously erroneous if you apply the formula to determine the speed of a reaction.arrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning  Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





