
It requires 799 kJ of energy to break one mole of carbon-oxygen double bonds in carbon dioxide. What
Interpretation:
The energy required to rupture one mole of
Concept introduction:
Bohr developed a rule for quantization of energy that could be applicable to the electron of an atom in motion. By using this he derived a formula for energy levels of electron in H-atom.
Relation between frequency and wavelength is,
C is the speed of light.
h is Planck’s constant (
E is energy of light particle.
The distance between any two similar points of a wave is called wavelength
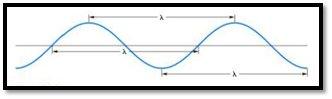
Figure 1
Frequency is defined as number of wavelengths of a wave that can pass through a point in one second.
Answer to Problem 7.108QP
The wavelength of light used to break per bond is
Explanation of Solution
To calculate: The wavelength of light used to break per bond.
The energy of each photon is calculated as follows,
The wave length of light used to break per bond is
The obtained wavelength is in range of near UV region of electromagnetic spectrum which corresponds to lyman series of H-atom. The minimum energy required in this region for transition corresponds to
The obtained energy is more than required energy to break the
By using the formula for energy levels of electron in H-atom, the wavelength of light used to break per bond was calculated.
Want to see more full solutions like this?
Chapter 7 Solutions
Bundle: General Chemistry, Loose-leaf Version, 11th + OWLv2, 4 terms (24 months) Printed Access Card
- A molecule shows peaks at 1379, 1327, 1249, 739 cm-1. Draw a diagram of the energy levels for such a molecule. Draw arrows for the possible transitions that could occur for the molecule. In the diagram imagine exciting an electron, what are its various options for getting back to the ground state? What process would promote radiation less decay? What do you expect for the lifetime of an electron in the T1 state? Why is phosphorescence emission weak in most substances? What could you do to a sample to enhance the likelihood that phosphorescence would occur over radiationless decay?arrow_forwardRank the indicated C—C bonds in increasing order of bond length. Explain as why to the difference.arrow_forwardUse IUPAC rules to name the following alkanearrow_forward
- Please correct answer and don't use hand ratingarrow_forwardPlease correct answer and don't use hand ratingarrow_forwardThe SN 1 mechanism starts with the rate-determining step which is the dissociation of the alkyl halide into a carbocation and a halide ion. The next step is the rapid reaction of the carbocation intermediate with the nucleophile; this step completes the nucleophilic substitution stage. The step that follows the nucleophilic substitution is a fast acid-base reaction. The nucleophile now acts as a base to remove the proton from the oxonium ion from the previous step, to give the observed product. Draw a curved arrow mechanism for the reaction, adding steps as necessary. Be sure to include all nonzero formal charges. Cl: Add/Remove step G Click and drag to start drawing a structure.arrow_forward
- Please correct answer and don't use hand ratingarrow_forwardA monochromatic light with a wavelength of 2.5x10-7m strikes a grating containing 10,000 slits/cm. Determine the angular positions of the second-order bright line.arrow_forwardCurved arrows are used to illustrate the flow of electrons. Us the reaction conditions provided and follow the curved arrow to draw the resulting structure(s). Include all lone pairs and charges as appropriate. H :I H 0arrow_forward
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning





