
Organic Chemistry As a Second Language: Second Semester Topics
4th Edition
ISBN: 9781119110651
Author: David R. Klein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 6.6, Problem 6.55P
Predict the product(s) for each of the following transformations:
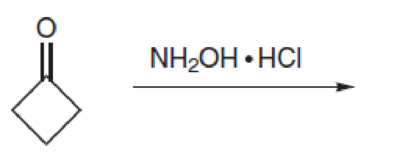
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Which of the ketones below could not be prepared by an acid-catalyzed hydration of an alkyne?
Propose a suitable synthesis to accomplish the following transformations. Please give the
reagents and products for every step. Do not just list the reagents
Propose an efficient synthesis (with mechanisms) for each of the following transformations:
b)
O
O
OH
O
CO₂H
Chapter 6 Solutions
Organic Chemistry As a Second Language: Second Semester Topics
Ch. 6.1 - Predict the major product for each of the...Ch. 6.1 - Predict the major product for each of the...Ch. 6.1 - Predict the major product for each of the...Ch. 6.1 - Predict the major product for each of the...Ch. 6.1 - Predict the major product for each of the...Ch. 6.1 - Predict the major product for each of the...Ch. 6.1 - Identify the reagents you would use to achieve...Ch. 6.1 - Identify the reagents you would use to achieve...Ch. 6.1 - Identify the reagents you would use to achieve...Ch. 6.1 - Identify the reagents you would use to achieve...
Ch. 6.3 - Predict the major product of each of the following...Ch. 6.3 - Predict the major product of each of the following...Ch. 6.3 - Predict the major product of each of the following...Ch. 6.3 - Predict the major product of each of the following...Ch. 6.3 - Predict the major product of each of the following...Ch. 6.3 - Propose a mechanism for each of the following...Ch. 6.3 - Propose a mechanism for each of the following...Ch. 6.3 - Propose a mechanism for each of the following...Ch. 6.4 - Prob. 6.24PCh. 6.4 - Prob. 6.25PCh. 6.4 - Prob. 6.26PCh. 6.4 - Prob. 6.27PCh. 6.4 - Predict the major product of each of the following...Ch. 6.4 - Predict the major product of each of the following...Ch. 6.4 - Predict the major product of each of the following...Ch. 6.4 - Predict the major product of each of the following...Ch. 6.5 - Prob. 6.33PCh. 6.5 - Prob. 6.34PCh. 6.5 - Prob. 6.35PCh. 6.5 - Predict the major product that is expected when...Ch. 6.5 - Prob. 6.37PCh. 6.5 - Predict the major product that is expected when...Ch. 6.5 - Identify the reagents you would use to achieve...Ch. 6.5 - Identify the reagents you would use to achieve...Ch. 6.5 - Identify the reagents you would use to achieve...Ch. 6.5 - Identify the reagents you would use to achieve...Ch. 6.5 - Identify the reagents you would use to achieve...Ch. 6.5 - Identify the reagents you would use to achieve...Ch. 6.5 - Identify the reagents you would use to achieve...Ch. 6.5 - Identify the reagents you would use to achieve...Ch. 6.6 - Predict the product(s) for each of the following...Ch. 6.6 - Predict the product(s) for each of the following...Ch. 6.6 - Predict the product(s) for each of the following...Ch. 6.6 - Predict the product(s) for each of the following...Ch. 6.6 - Predict the product(s) for each of the following...Ch. 6.6 - Predict the product(s) for each of the following...Ch. 6.6 - Predict the product(s) for each of the following...Ch. 6.6 - Predict the product(s) for each of the following...Ch. 6.6 - Predict the product(s) for each of the following...Ch. 6.6 - Predict the product(s) for each of the following...Ch. 6.6 - Predict the product(s) for each of the following...Ch. 6.6 - Predict the product(s) for each of the following...Ch. 6.6 - Predict the product(s) for each of the following...Ch. 6.7 - Prob. 6.62PCh. 6.7 - Prob. 6.63PCh. 6.7 - Prob. 6.64PCh. 6.7 - Prob. 6.65PCh. 6.7 - Prob. 6.67PCh. 6.7 - Prob. 6.68PCh. 6.7 - Prob. 6.69PCh. 6.7 - Predict the major product for each of the...Ch. 6.7 - Predict the major product for each of the...Ch. 6.7 - Predict the major product for each of the...Ch. 6.8 - Predict the major product for each of the...Ch. 6.8 - Predict the major product for each of the...Ch. 6.8 - Predict the major product for each of the...Ch. 6.8 - Predict the major product for each of the...Ch. 6.8 - Predict the major product for each of the...Ch. 6.9 - Prob. 6.81PCh. 6.9 - Prob. 6.82PCh. 6.9 - Prob. 6.83PCh. 6.9 - What reagents would you use to achieve the...Ch. 6.9 - What reagents would you use to achieve the...Ch. 6.9 - What reagents would you use to achieve the...Ch. 6.9 - What reagents would you use to achieve the...Ch. 6.9 - What reagents would you use to achieve the...Ch. 6.9 - What reagents would you use to achieve the...Ch. 6.9 - What reagents would you use to achieve the...Ch. 6.9 - What reagents would you use to achieve the...Ch. 6.9 - What reagents would you use to achieve the...Ch. 6.9 - What reagents would you use to achieve the...Ch. 6.9 - What reagents would you use to achieve the...Ch. 6.9 - What reagents would you use to achieve the...Ch. 6.9 - What reagents would you use to achieve the...Ch. 6.9 - What reagents would you use to achieve the...Ch. 6.9 - What reagents would you use to achieve the...Ch. 6.9 - What reagents would you use to achieve the...Ch. 6.9 - What reagents would you use to achieve the...Ch. 6.9 - Propose an efficient synthesis for the following...Ch. 6.9 - Propose an efficient synthesis for the following...Ch. 6.9 - Propose an efficient synthesis for the following...Ch. 6.9 - Propose an efficient synthesis for the following...Ch. 6.9 - Propose an efficient synthesis for the following...Ch. 6.9 - Propose an efficient synthesis for the following...Ch. 6.9 - Propose an efficient synthesis for the following...Ch. 6.9 - Propose an efficient synthesis for the following...Ch. 6.9 - Propose an efficient synthesis for the following...Ch. 6.9 - Propose an efficient synthesis for the following...
Additional Science Textbook Solutions
Find more solutions based on key concepts
How many electrons are in each energy level of the following elements? a. H b. F c. Ar d. K
General, Organic, and Biological Chemistry (3rd Edition)
Calculate the lattice energy of CaCl2 using a Born-Haber cycle and data from Appendices F and L and Table 7.5. ...
Chemistry & Chemical Reactivity
A compound that contains only C and H was burned in excess O2 to give CO2 and H2O. When 0.270 g of the compound...
General Chemistry: Atoms First
Correct any incorrect equations. If no reaction occurs, write NO REACTION. a. Ba(NO3)2(aq)+(NH4)2SO4(aq)BaSO4(s...
Introductory Chemistry (6th Edition)
Match the substance on the left with the basic units that compose them on the right Remember that atomic elemen...
Introductory Chemistry (5th Edition) (Standalone Book)
Name each of the following:
Organic Chemistry (8th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Cyclohexene can be converted to 1-cyclopentenecarbaldehyde by the following series of reactions. Propose a structural formula for each intermediate compound.arrow_forwardNonconjugated , -unsaturated ketones, such as 3-cyclohexenone, are in an acid-catalyzed equilibrium with their conjugated , -unsaturated isomers. Propose a mechanism for this isomerization.arrow_forwardPropose an efficient synthesis for each of the following transformations: a-darrow_forward
- Which of the alkenes below would yield a mixture of the two alcohols shown upon hydroboration? 1) BH3 , THF 2) H-О, NaOH alkene ноarrow_forwardThe hemiacetal reaction is also reversible and can also be catalyzed by either acid or base. Hemiacetals are not usually isolated, except in the formation of 5- and 6-membered rings, as often seen in carbohydrate chemistry. Identify the hemiacetal formed from the intramolecular cyclization of the molecule shown. OH OH NaOH ? H CH₂OH OH OH The hemiacetal product is: HOH₂C HO O HOH₂C H OH OH HO OH HO OH HO OH O HOH₂C О OH OH H HO- OH HO OH CH₂OH OH OHarrow_forwardWhich of the following pairs of compounds are keto-enol tautomers?arrow_forward
- Propose an efficient synthesis for each of the following transformations:arrow_forwardPredict the major organic product of each of the following reactions or provide the reagent needed to complete each transformation.arrow_forwardUsing cyclohexanone as the starting material, describe how the following compounds can be synthesized:arrow_forward
- Indicate reagents and conditions needed to impart the transformations below. You do not need to indicate the mechanism, each will require more then one-step.arrow_forwardProvide the reagent and mechanism for the transformation below. Explain in detail how the reaction conditions provide the given regioselectivity observed in the product.arrow_forwardAcid-catalyzed hydrolysis of the following epoxide gives a trans diol. CH3 CH3 CH3 НО, НО, H,SO, + H,O HO HO Only this glycol This glycol is is formed. not formed. Of the two possible trans diols, only one is formed. How do you account for this stereoselectivity?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Coenzymes and cofactors; Author: CH15 SWAYAM Prabha IIT Madras;https://www.youtube.com/watch?v=bubY2Nm7hVM;License: Standard YouTube License, CC-BY
Aromaticity and Huckel's Rule; Author: Professor Dave Explains;https://www.youtube.com/watch?v=7-BguH4_WBQ;License: Standard Youtube License