
Organic Chemistry As a Second Language: Second Semester Topics
4th Edition
ISBN: 9781119110651
Author: David R. Klein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 6.9, Problem 6.82P
Interpretation Introduction
Interpretation:
Reagent for the given conversion has to be predicted.
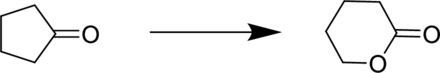
Concept Introduction:
Baeyer Villiger oxidation is an oxidation reaction in which the
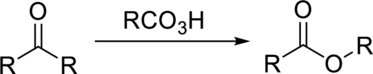
The rate of migration can be given as:
The commonly used Peroxy acids are meta-Chloroperoxybenzoic acid (MCPBA) and Peroxyacetic acid.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Draw the mechanism for the acid-catalyzed dehydration of 2-methyl-hexan-2-ol.
Draw the product of the reaction no mechanism required.
Identify the glycosidic linkage.
Chapter 6 Solutions
Organic Chemistry As a Second Language: Second Semester Topics
Ch. 6.1 - Predict the major product for each of the...Ch. 6.1 - Predict the major product for each of the...Ch. 6.1 - Predict the major product for each of the...Ch. 6.1 - Predict the major product for each of the...Ch. 6.1 - Predict the major product for each of the...Ch. 6.1 - Predict the major product for each of the...Ch. 6.1 - Identify the reagents you would use to achieve...Ch. 6.1 - Identify the reagents you would use to achieve...Ch. 6.1 - Identify the reagents you would use to achieve...Ch. 6.1 - Identify the reagents you would use to achieve...
Ch. 6.3 - Predict the major product of each of the following...Ch. 6.3 - Predict the major product of each of the following...Ch. 6.3 - Predict the major product of each of the following...Ch. 6.3 - Predict the major product of each of the following...Ch. 6.3 - Predict the major product of each of the following...Ch. 6.3 - Propose a mechanism for each of the following...Ch. 6.3 - Propose a mechanism for each of the following...Ch. 6.3 - Propose a mechanism for each of the following...Ch. 6.4 - Prob. 6.24PCh. 6.4 - Prob. 6.25PCh. 6.4 - Prob. 6.26PCh. 6.4 - Prob. 6.27PCh. 6.4 - Predict the major product of each of the following...Ch. 6.4 - Predict the major product of each of the following...Ch. 6.4 - Predict the major product of each of the following...Ch. 6.4 - Predict the major product of each of the following...Ch. 6.5 - Prob. 6.33PCh. 6.5 - Prob. 6.34PCh. 6.5 - Prob. 6.35PCh. 6.5 - Predict the major product that is expected when...Ch. 6.5 - Prob. 6.37PCh. 6.5 - Predict the major product that is expected when...Ch. 6.5 - Identify the reagents you would use to achieve...Ch. 6.5 - Identify the reagents you would use to achieve...Ch. 6.5 - Identify the reagents you would use to achieve...Ch. 6.5 - Identify the reagents you would use to achieve...Ch. 6.5 - Identify the reagents you would use to achieve...Ch. 6.5 - Identify the reagents you would use to achieve...Ch. 6.5 - Identify the reagents you would use to achieve...Ch. 6.5 - Identify the reagents you would use to achieve...Ch. 6.6 - Predict the product(s) for each of the following...Ch. 6.6 - Predict the product(s) for each of the following...Ch. 6.6 - Predict the product(s) for each of the following...Ch. 6.6 - Predict the product(s) for each of the following...Ch. 6.6 - Predict the product(s) for each of the following...Ch. 6.6 - Predict the product(s) for each of the following...Ch. 6.6 - Predict the product(s) for each of the following...Ch. 6.6 - Predict the product(s) for each of the following...Ch. 6.6 - Predict the product(s) for each of the following...Ch. 6.6 - Predict the product(s) for each of the following...Ch. 6.6 - Predict the product(s) for each of the following...Ch. 6.6 - Predict the product(s) for each of the following...Ch. 6.6 - Predict the product(s) for each of the following...Ch. 6.7 - Prob. 6.62PCh. 6.7 - Prob. 6.63PCh. 6.7 - Prob. 6.64PCh. 6.7 - Prob. 6.65PCh. 6.7 - Prob. 6.67PCh. 6.7 - Prob. 6.68PCh. 6.7 - Prob. 6.69PCh. 6.7 - Predict the major product for each of the...Ch. 6.7 - Predict the major product for each of the...Ch. 6.7 - Predict the major product for each of the...Ch. 6.8 - Predict the major product for each of the...Ch. 6.8 - Predict the major product for each of the...Ch. 6.8 - Predict the major product for each of the...Ch. 6.8 - Predict the major product for each of the...Ch. 6.8 - Predict the major product for each of the...Ch. 6.9 - Prob. 6.81PCh. 6.9 - Prob. 6.82PCh. 6.9 - Prob. 6.83PCh. 6.9 - What reagents would you use to achieve the...Ch. 6.9 - What reagents would you use to achieve the...Ch. 6.9 - What reagents would you use to achieve the...Ch. 6.9 - What reagents would you use to achieve the...Ch. 6.9 - What reagents would you use to achieve the...Ch. 6.9 - What reagents would you use to achieve the...Ch. 6.9 - What reagents would you use to achieve the...Ch. 6.9 - What reagents would you use to achieve the...Ch. 6.9 - What reagents would you use to achieve the...Ch. 6.9 - What reagents would you use to achieve the...Ch. 6.9 - What reagents would you use to achieve the...Ch. 6.9 - What reagents would you use to achieve the...Ch. 6.9 - What reagents would you use to achieve the...Ch. 6.9 - What reagents would you use to achieve the...Ch. 6.9 - What reagents would you use to achieve the...Ch. 6.9 - What reagents would you use to achieve the...Ch. 6.9 - What reagents would you use to achieve the...Ch. 6.9 - Propose an efficient synthesis for the following...Ch. 6.9 - Propose an efficient synthesis for the following...Ch. 6.9 - Propose an efficient synthesis for the following...Ch. 6.9 - Propose an efficient synthesis for the following...Ch. 6.9 - Propose an efficient synthesis for the following...Ch. 6.9 - Propose an efficient synthesis for the following...Ch. 6.9 - Propose an efficient synthesis for the following...Ch. 6.9 - Propose an efficient synthesis for the following...Ch. 6.9 - Propose an efficient synthesis for the following...Ch. 6.9 - Propose an efficient synthesis for the following...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the mechanism for the substitution reaction converting an alcohol into an alkyl halide. If chirality is important to the reaction include it.arrow_forwardWrite, in words three different reactions we can use to make an alcohol.arrow_forwardDraw the reduction mechanism for the reduction of the aldehyde.arrow_forward
- What is the product of the reaction of XeF4 with H2O? Group of answer choices H2XeF2 H2XeF4 XeO3 H2XeOarrow_forwardWhile noble gas exerts the strongest London (dispersion) forces on neighboring atoms? Group of answer choices Xe Ar Kr Nearrow_forwardWhich of the following elements is corrosive to your skin due to that element breaking down C=C bonds? Group of answer choices fluorine iodine bromine chlorinearrow_forward
- What the best source of sulfide to use on a small scale in the lab? Group of answer choices thiourea H2S NaHS Na2Sarrow_forwardWhich of the following statements about sulfur is FALSE? Group of answer choices H2S is the product of an oxygen-depleted ecosystem. In the acid mine drainage reaction, FeS2 is a product. One allotrope of sulfur has the formula S20. In the environment, bacterial oxidation can convert S2− to elemental S or SO42−.arrow_forwardOf the following choices, which is the best reason that most materials DON'T spontaneously combust even though our atmosphere is about 21% oxygen? Group of answer choices The reduction of O2 in the gas phase (O2 + e− → O2−) is spontaneous. The reduction of O2 in acid solution (O2 + H+ + e− → HO2(aq)) is spontaneous. O2 is not a reactant in combustion. The O2 bond dissociation energy is 494 kJ/mol, leading to a high activation energy for combustion.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning