
Organic Chemistry As a Second Language: Second Semester Topics
4th Edition
ISBN: 9781119110651
Author: David R. Klein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 6.3, Problem 6.21P
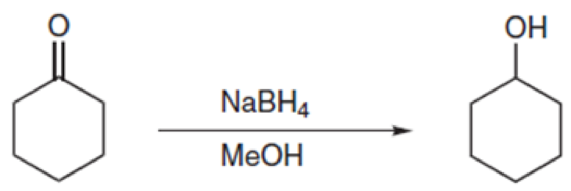
Propose a mechanism for each of the following transformations. The following problems will probably seem too easy—but just do them anyway. These basic arrows need to become routine for you, because we will step up the complexity in the next section, and you will want to have these basic skills down cold:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Draw a step-by-step mechanism for the transformation shown below (no additional reagents are needed), showing all electron flow with arrows.
Propose a plausible mechanism for the following transformation.
For the mechanism, draw the curved arrows as needed. Include lone pairs and charges in your answer. Do not draw out any hydrogen
explicitly in your products. Do not use abbreviations such as Me or Ph.
1) NaOH
LOH
2) H₂O
HO
5а. (
Show an SN2 mechanism for the following reaction. Include the transition
state, with stereochemistry. Use your work to predict the product, including
stereochemistry.
Br
H
acetone
5b.
predict the product, including stereochemistry.
Show an SN1 mechanism for the following reaction. Use your work to
HOʻ
Br
H
Chapter 6 Solutions
Organic Chemistry As a Second Language: Second Semester Topics
Ch. 6.1 - Predict the major product for each of the...Ch. 6.1 - Predict the major product for each of the...Ch. 6.1 - Predict the major product for each of the...Ch. 6.1 - Predict the major product for each of the...Ch. 6.1 - Predict the major product for each of the...Ch. 6.1 - Predict the major product for each of the...Ch. 6.1 - Identify the reagents you would use to achieve...Ch. 6.1 - Identify the reagents you would use to achieve...Ch. 6.1 - Identify the reagents you would use to achieve...Ch. 6.1 - Identify the reagents you would use to achieve...
Ch. 6.3 - Predict the major product of each of the following...Ch. 6.3 - Predict the major product of each of the following...Ch. 6.3 - Predict the major product of each of the following...Ch. 6.3 - Predict the major product of each of the following...Ch. 6.3 - Predict the major product of each of the following...Ch. 6.3 - Propose a mechanism for each of the following...Ch. 6.3 - Propose a mechanism for each of the following...Ch. 6.3 - Propose a mechanism for each of the following...Ch. 6.4 - Prob. 6.24PCh. 6.4 - Prob. 6.25PCh. 6.4 - Prob. 6.26PCh. 6.4 - Prob. 6.27PCh. 6.4 - Predict the major product of each of the following...Ch. 6.4 - Predict the major product of each of the following...Ch. 6.4 - Predict the major product of each of the following...Ch. 6.4 - Predict the major product of each of the following...Ch. 6.5 - Prob. 6.33PCh. 6.5 - Prob. 6.34PCh. 6.5 - Prob. 6.35PCh. 6.5 - Predict the major product that is expected when...Ch. 6.5 - Prob. 6.37PCh. 6.5 - Predict the major product that is expected when...Ch. 6.5 - Identify the reagents you would use to achieve...Ch. 6.5 - Identify the reagents you would use to achieve...Ch. 6.5 - Identify the reagents you would use to achieve...Ch. 6.5 - Identify the reagents you would use to achieve...Ch. 6.5 - Identify the reagents you would use to achieve...Ch. 6.5 - Identify the reagents you would use to achieve...Ch. 6.5 - Identify the reagents you would use to achieve...Ch. 6.5 - Identify the reagents you would use to achieve...Ch. 6.6 - Predict the product(s) for each of the following...Ch. 6.6 - Predict the product(s) for each of the following...Ch. 6.6 - Predict the product(s) for each of the following...Ch. 6.6 - Predict the product(s) for each of the following...Ch. 6.6 - Predict the product(s) for each of the following...Ch. 6.6 - Predict the product(s) for each of the following...Ch. 6.6 - Predict the product(s) for each of the following...Ch. 6.6 - Predict the product(s) for each of the following...Ch. 6.6 - Predict the product(s) for each of the following...Ch. 6.6 - Predict the product(s) for each of the following...Ch. 6.6 - Predict the product(s) for each of the following...Ch. 6.6 - Predict the product(s) for each of the following...Ch. 6.6 - Predict the product(s) for each of the following...Ch. 6.7 - Prob. 6.62PCh. 6.7 - Prob. 6.63PCh. 6.7 - Prob. 6.64PCh. 6.7 - Prob. 6.65PCh. 6.7 - Prob. 6.67PCh. 6.7 - Prob. 6.68PCh. 6.7 - Prob. 6.69PCh. 6.7 - Predict the major product for each of the...Ch. 6.7 - Predict the major product for each of the...Ch. 6.7 - Predict the major product for each of the...Ch. 6.8 - Predict the major product for each of the...Ch. 6.8 - Predict the major product for each of the...Ch. 6.8 - Predict the major product for each of the...Ch. 6.8 - Predict the major product for each of the...Ch. 6.8 - Predict the major product for each of the...Ch. 6.9 - Prob. 6.81PCh. 6.9 - Prob. 6.82PCh. 6.9 - Prob. 6.83PCh. 6.9 - What reagents would you use to achieve the...Ch. 6.9 - What reagents would you use to achieve the...Ch. 6.9 - What reagents would you use to achieve the...Ch. 6.9 - What reagents would you use to achieve the...Ch. 6.9 - What reagents would you use to achieve the...Ch. 6.9 - What reagents would you use to achieve the...Ch. 6.9 - What reagents would you use to achieve the...Ch. 6.9 - What reagents would you use to achieve the...Ch. 6.9 - What reagents would you use to achieve the...Ch. 6.9 - What reagents would you use to achieve the...Ch. 6.9 - What reagents would you use to achieve the...Ch. 6.9 - What reagents would you use to achieve the...Ch. 6.9 - What reagents would you use to achieve the...Ch. 6.9 - What reagents would you use to achieve the...Ch. 6.9 - What reagents would you use to achieve the...Ch. 6.9 - What reagents would you use to achieve the...Ch. 6.9 - What reagents would you use to achieve the...Ch. 6.9 - Propose an efficient synthesis for the following...Ch. 6.9 - Propose an efficient synthesis for the following...Ch. 6.9 - Propose an efficient synthesis for the following...Ch. 6.9 - Propose an efficient synthesis for the following...Ch. 6.9 - Propose an efficient synthesis for the following...Ch. 6.9 - Propose an efficient synthesis for the following...Ch. 6.9 - Propose an efficient synthesis for the following...Ch. 6.9 - Propose an efficient synthesis for the following...Ch. 6.9 - Propose an efficient synthesis for the following...Ch. 6.9 - Propose an efficient synthesis for the following...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Give Lewis dot structures and sketch the shapes of the following: a. SeCl4 b. l3- c. PSCI3 (P is central) d. lF...
Inorganic Chemistry
Without looking at the structures, give molecular formulas for the compounds in Problem 3-8 (a) and (b). Use th...
Organic Chemistry (9th Edition)
Determine [OH], [H+], and the pH of each of the following solutions. a. 1.0 M KCl b. 1.0 M KC2H3O2
Chemistry
ConceptualPRACTICE 2.1An element is a shiny, silver-colored solid at room temperature and pressure. It conducts...
Chemistry (7th Edition)
1.4 Consider the two spheres shown here, one made of silver and the other of aluminum.
What is the mass of eac...
Chemistry: The Central Science (13th Edition)
3. What are prefix multipliers? List some examples.
Chemistry: Structure and Properties (2nd Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1. Show how you could accomplish the following transformation. Be sure to show the structures of the products after each reaction you propose as well as all the reagents required for each reaction in the correct order. You do not need to show reaction mechanisms. НО 1arrow_forwardH₂, Pt This transformation can be performed with some reagent or combination of the reagents listed below. Give the necessary reagent(s) in the correct order, as a string of letters (without spaces or punctuation, such as "EBF"). There is more than one correct solution, so provide just one answer. A D TBAF HO Na, NH3 (1) B 1) NaNH,2) 3) H₂O* workup E H₂, Lindlar's cat. H OH NBS, hv C 1) NaNH ;2) NH: 2 l i 3) H3O* workup DMP or PCC TMSCI, Et Narrow_forwardShow how you could achieve the following transformation. Be sure to include all reagents and synthetic intermediates in your answer. You do not need to show mechanisms for the reactionsarrow_forward
- Using curved arrows to show electron movement, show the mechanisms for each of the reactions below.Include all formal charges, and do not use any reagents other than those given.arrow_forwardCurrent Attempt in Progress Provide a mechanism that explains formation of the following products. HBr + Br Br Your answer is partially correct. Draw step 1 of this mechanism. Include curved arrows only for reactants, lone pairs and formal charges in the mechanism. Draw out explicitly only the hydrogen of the HBr and do NOT include curved arrows for products. H₂C CH3 CH H-Br CH3 CH3 H₂C CH CH3 Edit Drawing The product of the above mechansism step has a resonance structure that leads to the formation of the thermodynamic (1,4- Addition) product. Draw ONLY the resonance structure of that molecule. Include formal charges in the structure and do NOT include any arrows. CH3 بہتر CH3 H₂C+arrow_forwardPropose an efficient synthesis for the given transformation. OH En "OMe This transformation can be performed with some reagent or combination of the reagents listed below. Give the necessary reagent(s) in the correct order, as a string of letters (without spaces or punctuation, such as "EBF"). If there is more than one correct solution, provide just one answer. A D E 1) &: 2) 1) &:2) H,0* t-BUOK 1) NaOMe; 2) H2, Lindlar's cat. H3O+ H3O* F G H 1) НCЕCNa; 2) NaH МСРВА Br2, hv HBr (xs), heat H3O* (RCO3H) K L M H2SO4, H20, HgSO4 MeONa DMP or PCC МеОн, Н2SO4 1) R2BH; 2) H2O2, NAOHarrow_forward
- Propose a mechanism that accounts for the following reaction. X Your answer is incorrect. Write the mechanism for step one of this reaction. Show lone pairs and formal charges. Only the acidic hydrogen should be drawn out with a covalent bond. Edit Drawingarrow_forwardHelp with 3 a and barrow_forwardWhen 2,2-dimethylcyclohexanol reacts with acid in water, multiple products form. One of these products is shown below. Provide a complete mechanism for the following transformation using only the reagents listed in the reaction scheme below.Show the intermediate(s) in the blank box(es) and draw all electrons and charges. In each box, only show one mechanistic transformation. Include only the reagents that are reacting at the time; do not show any byproducts.arrow_forward
- propose a mechanism for the reaction shown below. If the mechanism has more than one step, show each step separately. Show all charges and keep count of the electrons by the appropriate notation("curved arrow" or "fishhook" notation).arrow_forwardShow reagents that can be used to achieve the following transformation: A feasible synthetic pathway goes through the ketone shown below: & 1st reagents t-BUOK The transformations above can be performed with some reagent or combination of the reagents listed below. Give the necessary reagent(s) in the correct order for each transformation, as a string of letters (without spaces or punctuation, such as "EBF"). If there is more than one correct solution, provide just one answer. A B E H₂, Lindlar's cat. 1) LIAH 2) H₂O مل 4 2nd reagents conc. H₂SO4 heat F H₂ Pt DMP or PCC 4 C Na₂Cr₂O7 H₂SO H₂O G Bra, hv K 1) MeMgBr: 2) H₂O* D Na Me H dilute H₂SO4 L 1) BH, THF: 2) H₂O₂, NaOHarrow_forwardShow reagents that can be used to achieve the following transformation: A feasible synthetic pathway goes through the ketone shown below: 1st reagents مل 2nd reagents The transformations above can be performed with some reagent or combination of the reagents listed below. Give the necessary reagent(s) in the correct order for each transformation, as a string of letters (without spaces or punctuation, such as "EBF"). If there is more than one correct solution, provide just one answer. A t-BuOK B с D conc. H2SO4, heat Na2Cr2O7, H2SO4, H₂O NaOMe E F G H H2, Lindlar's cat. H2, Pt Br2, hv dilute H2SO4arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Lipids - Fatty Acids, Triglycerides, Phospholipids, Terpenes, Waxes, Eicosanoids; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=7dmoH5dAvpY;License: Standard YouTube License, CC-BY