
ORGANIC CHEMISTRY - LOOSELEAF W/CONNECT
6th Edition
ISBN: 9781266060144
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 6.5, Problem 12P
The equilibrium constant for the conversion of the axial to the equatorial conformation of methoxycyclohexane is
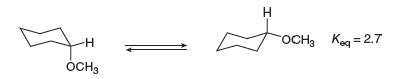
a. Given these data, which conformation is present in the larger amount at equilibrium?
b. Is
c. From the values in Table
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
3. Devise a retrosynthesis for the problem given below and then provide the corresponding
synthesis with all necessary reagents/reactants:
RETROSYNTHESIS:
SYNTHESIS:
Br
Several square planar complexes are known for Gold (III) ions but not for Silver (III) why?
Aiter running various experiments, you determine that the mechanism for the following reaction is bimolecular.
CI
Using this information, draw the correct mechanism in the space below.
X
Explanation
Check
C
Cl
OH + CI
Add/Remove step
Click and drag to start
drawing a structure.
2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy C
Chapter 6 Solutions
ORGANIC CHEMISTRY - LOOSELEAF W/CONNECT
Ch. 6.2 - Prob. 2PCh. 6.3 - Problem 6.3 By taking into account...Ch. 6.3 - Problem 6.4 Use curved arrows to show the movement...Ch. 6.3 - Problem 6.5 Follow the curved arrows and draw the...Ch. 6.4 - Prob. 6PCh. 6.4 - Problem 6.7 Use the values in Table 6.2 to...Ch. 6.4 - Prob. 8PCh. 6.5 - aWhich Keq corresponds to a negative value of G,...Ch. 6.5 - Given each of the following values, is the...Ch. 6.5 - Given each of the following values, is the...
Ch. 6.5 - The equilibrium constant for the conversion of the...Ch. 6.6 - Prob. 13PCh. 6.6 - For a reaction with H=40kJ/mol, decide which of...Ch. 6.6 - For a reaction with H=20kJ/mol, decide which of...Ch. 6.7 - Draw an energy diagram for a reaction in which the...Ch. 6.7 - Prob. 17PCh. 6.7 - Prob. 18PCh. 6.8 - Problem 6.19 Consider the following energy...Ch. 6.8 - Draw an energy diagram for a two-step reaction,...Ch. 6.9 - Which value if any corresponds to a faster...Ch. 6.9 - Prob. 22PCh. 6.9 - Problem 6.23 For each rate equation, what effect...Ch. 6.9 - Prob. 24PCh. 6.10 - Identify the catalyst in each equation. a....Ch. 6 - Draw the products of homolysis or heterolysis of...Ch. 6 - Explain why the bond dissociation energy for bond...Ch. 6 - Classify each transformation as substitution,...Ch. 6 - Prob. 29PCh. 6 - 6.31 (a) Add curved arrows for each step to show...Ch. 6 - Prob. 35PCh. 6 - 6.39. a. Which value corresponds to a negative...Ch. 6 - Prob. 40PCh. 6 - For which of the following reaction is S a...Ch. 6 - Prob. 42PCh. 6 - Prob. 43PCh. 6 - 6.44 Consider the following reaction: .
Use curved...Ch. 6 - Prob. 45PCh. 6 - 6.50 The conversion of acetyl chloride to methyl...Ch. 6 - Prob. 50PCh. 6 - Prob. 53P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Complete the reaction in the fewest number of steps as possible, Draw all intermediates (In the same form as the picture provided) and provide all reagents.arrow_forwardPlease provide steps to work for complete understanding.arrow_forwardPlease provide steps to work for complete understanding.arrow_forward
- Identify the Functional Groups (FG) in the following molecules. Classify C atoms as tertiary, 30, or quaternary 40. Identify secondary 20 and tertiary, 30 hydrogen atoms. Please provide steps to undertand each labeling.arrow_forwardIdentify the Functional Groups (FG) in the following molecules. Classify C atoms as tertiary, 30, or quaternary 40. Identify secondary 20 and tertiary, 30 hydrogen atoms. Please provide steps to undertand each labeling.arrow_forwardIdentify the Functional Groups (FG) in the following molecules. Classify C atoms as tertiary, 30, or quaternary 40. Identify secondary 20 and tertiary, 30 hydrogen atoms. Please provide steps to undertand each labeling.arrow_forward
- Identify the Functional Groups (FG) in the following molecules. Classify C atoms as tertiary, 30, or quaternary 40. Identify secondary 20 and tertiary, 30 hydrogen atoms. Please provide steps to undertand each labeling.arrow_forwardA certain chemical reaction releases 24.7 kJ/g of heat for each gram of reactant consumed. How can you calculate what mass of reactant will produce 1460. J of heat? Set the math up. But don't do any of it. Just leave your answer as a math expression. Also, be sure your answer includes all the correct unit symbols. mass M 0.0 x μ 00 1 Garrow_forwardPlease don't used hand raiting and don't used Ai solutionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
The Laws of Thermodynamics, Entropy, and Gibbs Free Energy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=8N1BxHgsoOw;License: Standard YouTube License, CC-BY