
ORG CHEM CONNECT CARD
6th Edition
ISBN: 9781264860746
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 6.5, Problem 12P
The equilibrium constant for the conversion of the axial to the equatorial conformation of methoxycyclohexane is
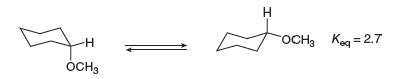
a. Given these data, which conformation is present in the larger amount at equilibrium?
b. Is
c. From the values in Table
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Pt
+ H₂
Draw the molecule on the canvas by choosing buttons from the Tools (for bonds), Atoms, and Advanced Templ
9 2 0 ©
120
Complete boxes in the flow chart. Draw the structure of the organic compound foundin each layer after adding 3M NaOH and extraction. Make sure to include any charges. Provide explanation on answers.
==
Vid4Q2
Unanswered
☑
Provide IUPAC name of product in the reaction
below
A 3,4-dimethylcyclohexene
B 1,2-dimethylcyclohexane
C 1,2-dimethylcyclohexene
D 3,4-dimethylcyclohexane
H₂
Pd
Chapter 6 Solutions
ORG CHEM CONNECT CARD
Ch. 6.2 - Prob. 2PCh. 6.3 - Problem 6.3 By taking into account...Ch. 6.3 - Problem 6.4 Use curved arrows to show the movement...Ch. 6.3 - Problem 6.5 Follow the curved arrows and draw the...Ch. 6.4 - Prob. 6PCh. 6.4 - Problem 6.7 Use the values in Table 6.2 to...Ch. 6.4 - Prob. 8PCh. 6.5 - aWhich Keq corresponds to a negative value of G,...Ch. 6.5 - Given each of the following values, is the...Ch. 6.5 - Given each of the following values, is the...
Ch. 6.5 - The equilibrium constant for the conversion of the...Ch. 6.6 - Prob. 13PCh. 6.6 - For a reaction with H=40kJ/mol, decide which of...Ch. 6.6 - For a reaction with H=20kJ/mol, decide which of...Ch. 6.7 - Draw an energy diagram for a reaction in which the...Ch. 6.7 - Prob. 17PCh. 6.7 - Prob. 18PCh. 6.8 - Problem 6.19 Consider the following energy...Ch. 6.8 - Draw an energy diagram for a two-step reaction,...Ch. 6.9 - Which value if any corresponds to a faster...Ch. 6.9 - Prob. 22PCh. 6.9 - Problem 6.23 For each rate equation, what effect...Ch. 6.9 - Prob. 24PCh. 6.10 - Identify the catalyst in each equation. a....Ch. 6 - Draw the products of homolysis or heterolysis of...Ch. 6 - Explain why the bond dissociation energy for bond...Ch. 6 - Classify each transformation as substitution,...Ch. 6 - Prob. 29PCh. 6 - 6.31 (a) Add curved arrows for each step to show...Ch. 6 - Prob. 35PCh. 6 - 6.39. a. Which value corresponds to a negative...Ch. 6 - Prob. 40PCh. 6 - For which of the following reaction is S a...Ch. 6 - Prob. 42PCh. 6 - Prob. 43PCh. 6 - 6.44 Consider the following reaction: .
Use curved...Ch. 6 - Prob. 45PCh. 6 - 6.50 The conversion of acetyl chloride to methyl...Ch. 6 - Prob. 50PCh. 6 - Prob. 53P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 5. Use the MS data to answer the questions on the next page. 14.0 1.4 15.0 8.1 100- MS-IW-5644 26.0 2.8 27.0 6.7 28.0 1.8 29.0 80 4.4 38.0 1.0 39.0 1.5 41.0 1.2 42.0 11.2 43.0 100.0 44.0 4.3 79.0 1.9 80.0 2.6 Relative Intensity 40 81.0 1.9 82.0 2.5 93.0 8.7 20- 95.0 8.2 121.0 2.0 123.0 2.0 136.0 11.8 0 138.0 11.5 20 40 8. 60 a. Br - 0 80 100 120 140 160 180 200 220 m/z Identify the m/z of the base peak and molecular ion. 2 b. Draw structures for each of the following fragments (include electrons and charges): 43.0, 93.0, 95.0, 136.0, and 138.0 m/z. C. Draw a reasonable a-fragmentation mechanism for the fragmentation of the molecular ion to fragment 43.0 m/z. Be sure to include all electrons and formal charges. 6. Using the values provided in Appendix E of your lab manual, calculate the monoisotopic mass for the pyridinium ion (CsH6N) and show your work.arrow_forwardNonearrow_forwardStereochemistry: Three possible answers- diastereomers, enantiomers OH CH₂OH I -c=0 21108 1101 41745 HOR CH₂OH IL Но CH₂OH TIL a. Compounds I and III have this relationship with each other: enantiomers b. Compounds II and IV have this relationship with each other: c. Compounds I and II have this relationship with each other: d. *Draw one structure that is a stereoisomer of II, but neither a diastereomer nor an enantiomer. (more than one correct answer)arrow_forward
- Don't used Ai solutionarrow_forwardDon't used Ai solutionarrow_forwardIn mass spectrometry, alpha cleavages are common in molecules with heteroatoms. Draw the two daughter ions that would be observed in the mass spectrum resulting from an alpha cleavage of this molecule. + NH2 Q Draw Fragment with m/z of 72arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning
The Laws of Thermodynamics, Entropy, and Gibbs Free Energy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=8N1BxHgsoOw;License: Standard YouTube License, CC-BY