
Concept explainers
Draw the products of homolysis or heterolysis of each indicated bond. Use electronegativity
differences to decide on the location of charges in the heterolysis reaction. Classify each
carbon reactive intermediate as a radical, carbocation, or carbanion.
a.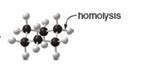 b.
b. 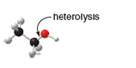
(a)
Interpretation: The products of homolysis or heterolysis of the indicated bond is to be drawn by using the electronegativity differences. Each carbon reactive intermediate is to be classified as a radical, carbocation, or carbanion.
Concept introduction: In organic chemistry, the formation of carbocation or carbanion occurs due to the heterolysis or homolysis process. Carbocations possess six electrons around them, whereas carbanions possess the lone pair of electrons. Carbocation behaves as electrophile due to lack of electrons and incomplete octet.
Answer to Problem 26P
In the given indicated bond, homolysis takes place that results in the formation of the radicals.
The first product is,
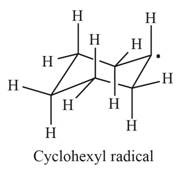
The second product is,

Explanation of Solution
The heterolysis does not take place in the given compound due to the less electronegativity difference between atoms.
The product of homolysis is shown below.
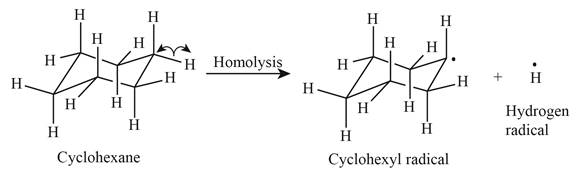
Figure 1
In the above reaction, cyclohexane forms cyclohexyl radical and hydrogen radical by homolysis. Homolysis is opposite to the heterolysis. It forms radical with unpaired electron because the electrons are not attracted toward one element in the homolysis.
In the given indicated bond, homolysis takes place that results in the formation of the radical. The product of homolysis is shown in Figure 1.
(b)
Interpretation: The products of homolysis or heterolysis of the indicated bond is to be drawn by using the electronegativity differences. Each carbon reactive intermediate is to be classified as a radical, carbocation, or carbanion.
Concept introduction: In organic chemistry, the formation of carbocation or carbanion occurs due to the heterolysis or homolysis process. Carbocations possess six electrons around them, whereas carbanions possess the lone pair of electrons. Carbocation behaves as electrophile due to lack of electrons and incomplete octet.
Carbanion behaves as a nucleophile in the chemical reaction due to the presence of excess electrons. Heterolysis is the process in which unequal sharing of electrons results in the breaking of the bond.
Answer to Problem 26P
In the given indicated bond, heterolysis takes place that results in the formation of the carbocation.
The first product is,
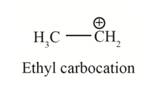
The second product is,
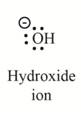
Explanation of Solution
Heterolysis in the compound takes place due to the more electronegativity difference. The product of heterolysis is shown below.
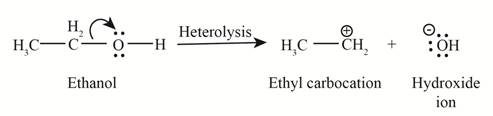
Figure 2
In the above reaction, ethanol forms ethyl carbocation and hydroxide ion by heterolysis. The heterolysis in the chemical reaction leads to the formation of ionic species because electrons are attracted toward more electronegative atom. Oxygen is more electronegative than carbon. Therefore, heterolysis and the formation of carbocation take place in the reaction.
In the given indicated bond, heterolysis takes place that results in the formation of the carbocation. The product of heterolysis is shown in Figure 2.
Want to see more full solutions like this?
Chapter 6 Solutions
ORG CHEM CONNECT CARD
- Can you please help me with drawing the Lewis structure of each molecular formula?I truly appreciate you!arrow_forwardCan you please help me with drawing the Lewis structure of each molecular formula?I truly appreciate you!arrow_forwardCan you please help me with drawing the Lewis structure of each molecular formula?I truly appreciate you!arrow_forward
- Can you please help me with drawing the Lewis structure of each molecular formula?I truly appreciate you!arrow_forwardPlease draw and explainarrow_forwardDescribe each highlighted bond in terms of the overlap of atomic orbitals. (a) Н Н H H [References] HIC H H C H H-C-CC-N: H σ character n character (b) HIC H H H H-C-C-C HIC H Н H O-H σ character n character Submit Answer Try Another Version 3 item attempts remainingarrow_forward
- 11 Naming and drawing alcohols Write the systematic (IUPAC) name for each of the following organic molecules: structure OH HO OH Explanation Check name ☐arrow_forwardwhat is the drawn mechanism for diethyl carbonate and 4 - bromo - N, N -dimethylaniline to create crystal violet?arrow_forwardWhich of the following compounds are constitutional isomers of each other? I and II O II and III O III and IV OI and IV O II and IV CI H CI H CI H H CI H-C-C-CI C-C-C-CI H-C-C-CI H-C-C-CI H CI Ĥ ĆI A A Ĥ ĆI || IVarrow_forward
- Please correct answer and don't used hand raitingarrow_forwardQ1: Curved Arrows, Bronsted Acids & Bases, Lewis Acids & Bases Considering the following reactions: a) Predict the products to complete the reactions. b) Use curved electron-pushing arrows to show the mechanism for the reaction in the forward direction. Redraw some of the compounds to explicitly illustrate all bonds that are broken and all bonds that are formed. c) Label Bronsted acids and bases in the left side of the reactions. Label conjugate acids and bases in the right side of the reactions. d) Label Lewis acids and bases, nucleophiles and electrophiles in the left side of the reactions. A. + OH CH30: OH B. + HBr C. H₂SO4 D. CF 3. CH 3 + HCI N H fluoxetine antidepressant 1↓ JDownloadarrow_forwardDon't used Ai solutionarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
