
ORG CHEM CONNECT CARD
6th Edition
ISBN: 9781264860746
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 6.3, Problem 4P
Use curved arrows to show the movement of electrons in each equation.
a.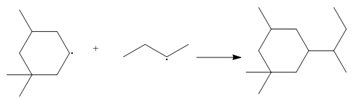
b.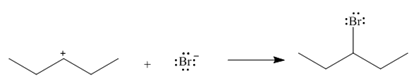
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Zeolites: environmental applications.
" is
The structure of the bicarbonate (hydrogen carbonate) ion, HCO3-, HCO3
best described as a hybrid of several contributing resonance forms, two of which
are shown here.
HO
:0:
:Ö:
HO
+
Bicarbonate is crucial for the control of body pH (for example, blood pH:
7.4). A more self-indulgent use is in baking soda, where it serves as a
source of CO2 CO₂ 2 gas, which gives bread and pastry their fluffy
constituency.
(i) Draw at least one additional resonance form.
=
(ii) Using curved "electron-pushing" arrows, show how these Lewis structures may
be interconverted by movement of electron pairs. (iii) Determine which form or
forms will be the major contributor(s) to the real structure of bicarbonate,
explaining your answer on the basis of the criteria in Section 1-5.
Which of these is the best use of a volumetric flask?
measuring how much liquid it contains
delivering a precise amount of liquid to another container
holding solutions
making solutions of precise concentration
Chapter 6 Solutions
ORG CHEM CONNECT CARD
Ch. 6.2 - Prob. 2PCh. 6.3 - Problem 6.3 By taking into account...Ch. 6.3 - Problem 6.4 Use curved arrows to show the movement...Ch. 6.3 - Problem 6.5 Follow the curved arrows and draw the...Ch. 6.4 - Prob. 6PCh. 6.4 - Problem 6.7 Use the values in Table 6.2 to...Ch. 6.4 - Prob. 8PCh. 6.5 - aWhich Keq corresponds to a negative value of G,...Ch. 6.5 - Given each of the following values, is the...Ch. 6.5 - Given each of the following values, is the...
Ch. 6.5 - The equilibrium constant for the conversion of the...Ch. 6.6 - Prob. 13PCh. 6.6 - For a reaction with H=40kJ/mol, decide which of...Ch. 6.6 - For a reaction with H=20kJ/mol, decide which of...Ch. 6.7 - Draw an energy diagram for a reaction in which the...Ch. 6.7 - Prob. 17PCh. 6.7 - Prob. 18PCh. 6.8 - Problem 6.19 Consider the following energy...Ch. 6.8 - Draw an energy diagram for a two-step reaction,...Ch. 6.9 - Which value if any corresponds to a faster...Ch. 6.9 - Prob. 22PCh. 6.9 - Problem 6.23 For each rate equation, what effect...Ch. 6.9 - Prob. 24PCh. 6.10 - Identify the catalyst in each equation. a....Ch. 6 - Draw the products of homolysis or heterolysis of...Ch. 6 - Explain why the bond dissociation energy for bond...Ch. 6 - Classify each transformation as substitution,...Ch. 6 - Prob. 29PCh. 6 - 6.31 (a) Add curved arrows for each step to show...Ch. 6 - Prob. 35PCh. 6 - 6.39. a. Which value corresponds to a negative...Ch. 6 - Prob. 40PCh. 6 - For which of the following reaction is S a...Ch. 6 - Prob. 42PCh. 6 - Prob. 43PCh. 6 - 6.44 Consider the following reaction: .
Use curved...Ch. 6 - Prob. 45PCh. 6 - 6.50 The conversion of acetyl chloride to methyl...Ch. 6 - Prob. 50PCh. 6 - Prob. 53P
Additional Science Textbook Solutions
Find more solutions based on key concepts
Separate the list P,F,V,,T,a,m,L,t, and V into intensive properties, extensive properties, and nonproperties.
Fundamentals Of Thermodynamics
More than one choice may apply. Using the terms listed below, fill in the blank with the proper term. anterior ...
Essentials of Human Anatomy & Physiology (12th Edition)
To test your knowledge, discuss the following topics with a study partner or in writing ideally from memory. Th...
HUMAN ANATOMY
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
Some people compare DNA to a blueprint stored in the office of a construction company. Explain how this analogy...
Biology: Concepts and Investigations
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- You're competing on a Great British television game show, and you need to bake a cake. The quantity for each ingredient is given in grams, but you haven't been given a kitchen scale. Which of these properties would correlate with the mass of a baking ingredient like eggs or milk? Check all that apply. depth of color viscosity volume densityarrow_forwardDraw a Lewis structure for each of the following species. Again, assign charges where appropriate. a. H-H¯ b. CH3-CH3 c. CH3+CH3 d. CH3 CH3 e. CH3NH3+CH3NH3 f. CH30-CH3O¯ g. CH2CH2 - h. HC2-(HCC) HC2 (HCC) i. H202×(HOOH) H₂O₂ (HOOH) Nortonarrow_forwardIs molecule 6 an enantiomer?arrow_forward
- Show work. Don't give Ai generated solutionarrow_forwardCheck the box under each structure in the table that is an enantiomer of the molecule shown below. If none of them are, check the none of the above box under the table. Molecule 1 Molecule 2 Molecule 3 ----||| Molecule 4 Molecule 5 Molecule 6 none of the above mm..arrow_forwardShow work. don't give Ai generated solutionarrow_forward
- Check the box under each structure in the table that is an enantiomer of the molecule shown below. If none of them are, check the none of the above box under the table. Molecule 1 Molecule 2 Molecule 3 ----||| Molecule 4 Molecule 5 Molecule 6 none of the above mm..arrow_forwardUse the vapor-liquid equilibrium data at 1.0 atm. for methanol-water (Table 2-8 ) for the following: If the methanol vapor mole fraction is 0.600, what is the methanol liquid mole fraction? Is there an azeotrope in the methanol-water system at a pressure of 1.0 atmospheres? If water liquid mole fraction is 0.350, what is the water vapor mole fraction? What are the K values of methanol and of water at a methanol mole fraction in the liquid of 0.200? What is the relative volatility αM-W at a methanol mole fraction in the liquid of 0.200?arrow_forwardCheck the box under each structure in the table that is an enantiomer of the molecule shown below. If none of them are, check the none of the above box under the table. || |II***** Molecule 1 | Molecule 4 none of the above Molecule 2 Molecule 3 Х mm... C ---||| *** Molecule 5 Molecule 6arrow_forward
- is SiBr4 Silicon (IV) tetra Bromine? is KClO2 potassium dihypochlorite ?arrow_forward"יוון HO" Br CI Check the box under each structure in the table that is an enantiomer of the molecule shown below. If none of them are, check the none of the above box under the table. Molecule 1 Molecule 2 Molecule 3 Br Br Br HO OH H CI OH ✓ Molecule 4 Molecule 5 Molecule 6 CI Br יייון H Br OH OH CI Br ☐ none of the above × Garrow_forwardUS2 Would this be Uranium (II) diSulfide?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

GCSE Chemistry - Differences Between Compounds, Molecules & Mixtures #3; Author: Cognito;https://www.youtube.com/watch?v=jBDr0mHyc5M;License: Standard YouTube License, CC-BY