
(a)
Interpretation:
The stereoisomer products for the given reaction should be determined.
Concept introduction:
Nucleophile: Nucleophiles are electron rich compounds which donates electrons to electrophilic compounds which results in bond formation.
Electrophile: Electrophiles are electron deficient compounds which accepts electrons from nucleophiles that results in bond formation.
Electrophilic addition: It is a type of addition reaction in which the pi bond present in the molecule breaks as the electrophile approaches and results in the formation of product with sigma bond.
In addition reaction of
Oxidation Reaction: It involves loss of electrons, addition of oxygen atoms or removal of hydrogen atoms.
E configuration: The geometric isomers are given E configuration if high priority groups are placed on opposite sides of the bond.
Z configuration: The geometric isomers are given Z configuration if high priority groups are placed on same sides of the bond.
Stereo specific: The reaction is considered as stereo specific if the reactant is stereo isomers that give rise to different set of stereo isomers.
Stereoisomers: Two compounds with same molecular formula but different in their orientation are considered as isomers.
The presence of atom with non-super impossible mirror image is defined as enantiomers which are given
(b)
Interpretation:
The stereoisomer products for the given reaction should be determined.
Concept introduction:
Nucleophile: Nucleophiles are electron rich compounds which donates electrons to electrophilic compounds which results in bond formation.
Electrophile: Electrophiles are electron deficient compounds which accepts electrons from nucleophiles that results in bond formation.
Electrophilic addition: It is a type of addition reaction in which the pi bond present in the molecule breaks as the electrophile approaches and results in the formation of product with sigma bond.
In addition reaction of alkenes when two substituents approaches same side of
Oxidation Reaction: It involves loss of electrons, addition of oxygen atoms or removal of hydrogen atoms.
E configuration: The geometric isomers are given E configuration if high priority groups are placed on opposite sides of the bond.
Z configuration: The geometric isomers are given Z configuration if high priority groups are placed on same sides of the bond.
Stereo specific: The reaction is considered as stereo specific if the reactant is stereo isomers that give rise to different set of stereo isomers.
Stereoisomers: Two compounds with same molecular formula but different in their orientation are considered as isomers.
The presence of atom with non-super impossible mirror image is defined as enantiomers which are given
(c)
Interpretation:
The stereoisomer products for the given reaction should be determined.
Concept introduction:
Nucleophile: Nucleophiles are electron rich compounds which donates electrons to electrophilic compounds which results in bond formation.
Electrophile: Electrophiles are electron deficient compounds which accepts electrons from nucleophiles that results in bond formation.
Electrophilic addition: It is a type of addition reaction in which the pi bond present in the molecule breaks as the electrophile approaches and results in the formation of product with sigma bond.
Oxidation Reaction: It involves loss of electrons, addition of oxygen atoms or removal of hydrogen atoms.
Acid Catalyzed Hydration Reaction: The reaction involves breaking of phi bonds between carbon-carbon multiple bonds and addition of alcohol to more substituted position of carbon in the molecule.
First step is the acid donates proton to the alkene which leads to the formation of more stable carbo cation.
Then, the water is added to the given alkene through acid catalyzed reaction where the water gets added to the carbo cation finally, the removal of one proton from oxonium ion (oxygen with one positive charge) using water results in the formation of product.
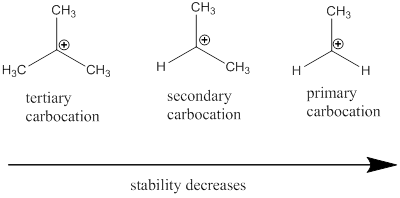
Carbocation: it is carbon ion that bears a positive charge on it.
Carbocation stability order:
(d)
Interpretation:
The stereoisomer products for the given reaction should be determined.
Concept introduction:
Nucleophile: Nucleophiles are electron rich compounds which donates electrons to electrophilic compounds which results in bond formation.
Electrophile: Electrophiles are electron deficient compounds which accepts electrons from nucleophiles that results in bond formation.
Electrophilic addition: It is a type of addition reaction in which the pi bond present in the molecule breaks as the electrophile approaches and results in the formation of product with sigma bond.
In addition reaction of alkenes when two substituents approaches same side of
Oxidation Reaction: It involves loss of electrons, addition of oxygen atoms or removal of hydrogen atoms.
E configuration: The geometric isomers are given E configuration if high priority groups are placed on opposite sides of the bond.
Z configuration: The geometric isomers are given Z configuration if high priority groups are placed on same sides of the bond.
Stereo specific: The reaction is considered as stereo specific if the reactant is stereo isomers that give rise to different set of stereo isomers.
Stereoisomers: Two compounds with same molecular formula but different in their orientation are considered as isomers.
The presence of atom with non-super impossible mirror image is defined as enantiomers which are given
(e)
Interpretation:
The stereoisomer products for the given reaction should be determined.
Concept introduction:
Nucleophile: Nucleophiles are electron rich compounds which donates electrons to electrophilic compounds which results in bond formation.
Electrophile: Electrophiles are electron deficient compounds which accepts electrons from nucleophiles that results in bond formation.
Electrophilic addition: It is a type of addition reaction in which the pi bond present in the molecule breaks as the electrophile approaches and results in the formation of product with sigma bond.
In addition reaction of alkenes when two substituents approaches same side of
Oxidation Reaction: It involves loss of electrons, addition of oxygen atoms or removal of hydrogen atoms.
E configuration: The geometric isomers are given E configuration if high priority groups are placed on opposite sides of the bond.
Z configuration: The geometric isomers are given Z configuration if high priority groups are placed on same sides of the bond.
Stereo specific: The reaction is considered as stereo specific if the reactant is stereo isomers that give rise to different set of stereo isomers.
Stereoisomers: Two compounds with same molecular formula but different in their orientation are considered as isomers.
The presence of atom with non-super impossible mirror image is defined as enantiomers which are given
(f)
Interpretation:
The stereoisomer products for the given reaction should be determined.
Concept introduction:
Nucleophile: Nucleophiles are electron rich compounds which donates electrons to electrophilic compounds which results in bond formation.
Electrophile: Electrophiles are electron deficient compounds which accepts electrons from nucleophiles that results in bond formation.
Electrophilic addition: It is a type of addition reaction in which the pi bond present in the molecule breaks as the electrophile approaches and results in the formation of product with sigma bond.
In addition reaction of alkenes when two substituents approaches same side of
Oxidation Reaction: It involves loss of electrons, addition of oxygen atoms or removal of hydrogen atoms.
E configuration: The geometric isomers are given E configuration if high priority groups are placed on opposite sides of the bond.
Z configuration: The geometric isomers are given Z configuration if high priority groups are placed on same sides of the bond.
Stereo specific: The reaction is considered as stereo specific if the reactant is stereo isomers that give rise to different set of stereo isomers.
Stereoisomers: Two compounds with same molecular formula but different in their orientation are considered as isomers.
The presence of atom with non-super impossible mirror image is defined as enantiomers which are given
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
Organic Chemistry; Organic Chemistry Study Guide A Format: Kit/package/shrinkwrap
- I apologize, but the app is not allowing me to post the other 4 pictures of the thermodynamics chart. But I believe the values are universal. Please help!arrow_forwardCalculating the pH of a salt solution Calculate the pH at 25 °C of a 0.29M solution of potassium butanoate (KC3H,CO2). Note that butanoic acid (HC3H,CO2) is a weak acid with a pKa of 4.82. Round your answer to 1 decimal place. pH = -0 Х olo 18 Ararrow_forward: At a certain temperature, the equilibrium constant K for the following reaction is 1.58 × 10-12 N2(g) + O2(g) = 2 NO(g) Use this information to complete the following table. Suppose a 38. L reaction vessel is filled with 0.93 mol of N2 and 0.93 mol of O2. What can you say about the composition of the mixture in the vessel at equilibrium? There will be very little N2 and O2. There will be very little NO. What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. 2 NO(g) N2(9)+02(9) What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. 3 N2(9)+302(g) 6 NO(g) Neither of the above is true. K = ☐ K = ☐ ☐ X10 Х D ? 000 18 Ar Barrow_forward
- when performing the reaction that involves 2 equivalents of 3-(diethylamino)-phenol and Phthalic anhydride with sulfuric acid and water react to form rhodamine b where the Phthalic anhydride cleaves in acid and how does Excessive Washing (w/ Base) & Subsequent Resonance Structure get affectedarrow_forward3. The strongest acid of the following compounds is ___.A. p-nitrophenol; B. m-nitrophenol; C. o-chlorophenol;D. p-methoxyphenol; E. o-methylphenol Please explain your steps and thought process. Thank you!arrow_forwardUsing the general properties of equilibrium constants At a certain temperature, the equilibrium constant K for the following reaction is 1.3 × 10 4: Cl2(g) + CHCl3(g) HCl(g) + CC₁(g) Use this information to complete the following table. Suppose a 16. L reaction vessel is filled with 1.6 mol of HCI and 1.6 mol of CCl4. What can you say about the composition of the mixture in the vessel at equilibrium? There will be very little Cl2 and CHCl3. ☐ x10 There will be very little HCI and CCl4. Neither of the above is true. What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. HCl(g)+CC14(g) 12 Cl2(9)+CHCl3(9) K = 0 ☐ What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. 2 Cl₂(9)+2CHCl3(9) 2 HCl(9)+2CC₁₁(9) K = ✓ 00. 18 Ararrow_forward
- 10. The most important reason why Br- is a better nucleophile than Cl-is ___. A. polarizability; B. size; C. solvation; D. basicity; E. polarity. Please include all steps. Thanks!arrow_forwardPredicting the qualitative acid-base properties of salts Consider the following data on some weak acids and weak bases: base acid Ка K₁₁ name formula name formula nitrous acid HNO2 4.5×10 4 pyridine CHEN 1.7 × 10 9 4 hydrofluoric acid HF 6.8 × 10 methylamine CH3NH2 | 4.4 × 10¯ Use this data to rank the following solutions in order of increasing pH. In other words, select a '1' next to the solution that will have the lowest pH, a '2' next to the solution that will have the next lowest pH, and so on. solution 0.1 M NaNO2 0.1 M KF pH choose one v choose one v 0.1 M C5H5NHBr 0.1 M CH3NH3CI choose one v ✓ choose one 1 (lowest) 2 ☑ 3 4 (highest) 000 18 Ararrow_forward4. The major product from treatment of 2-propanol with the Jonesreagent is ___.A. acetone; B. none of the other answers is correct C. propene; D.propanoic acid; E carbon dioxide. Please include all steps! Thank you!arrow_forward
- 7. All of the following compounds that are at the same oxidation levelare ___.u. methyl epoxide, v. propyne, w. propanal, x. propene,y. 2,2-dihydroxypropane, z. isopropanol?A. u,v,w,y; B. u,v,w; C. v,w,y,z; D. v, z; E. x,y,z Please include all steps. Thank you!arrow_forward9. Which one of the following substituents is the worst leaving group inan SN2 reaction? A. -NH2; B. -OH; C. –F; D. NH3; E. H2O Please include all steps. Thanks!arrow_forwardUsing the general properties of equilibrium constants At a certain temperature, the equilibrium constant K for the following reaction is 2.5 × 105: CO(g) + H2O(g) CO2(g) + H2(g) Use this information to complete the following table. Suppose a 7.0 L reaction vessel is filled with 1.7 mol of CO and 1.7 mol of H2O. What can you say about the composition of the mixture in the vessel at equilibrium? What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. CO2(9)+H2(g) CO(g)+H₂O(g) What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. 3 CO(g)+3H2O(g) = 3 CO2(g)+3H2(g) There will be very little CO and H2O. x10 There will be very little CO2 and H2. 000 Neither of the above is true. K = ☐ K = ☐ 18 Ararrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





