
Chemistry for Engineering Students
4th Edition
ISBN: 9781337398909
Author: Lawrence S. Brown, Tom Holme
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 6, Problem 6.83PAE
6.83 The graph below shows the first three ionization energies for sodium, magnesium, and aluminum. Without consulting a list of values, determine which line in the graph corresponds to each element.
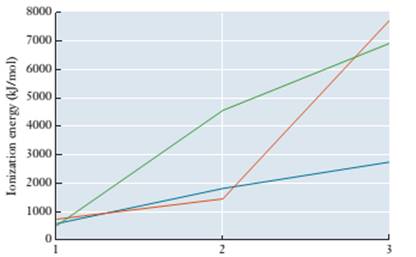
Number of electrons removed
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
The Periodic Table shows the common charges of ions that group elements form.
Using the information in the Periodic Table, how would you rank the elements rubidium (Rb), antimony (Sb), and iodine (I) in order of increasing number of valence electrons?
A
Antimony has a greater number of valence electrons than iodine but a lower number than rubidium.
B
Antimony has a lower number of valence electrons than iodine but a greater number than rubidium.
C
Iodine has a lower number of valence electrons than rubidium but a greater number than antimony.
D
Rubidium has a greater number of valence electrons than antimony but a lower number than iodine.
3.
4
Chapter 6 Solutions
Chemistry for Engineering Students
Ch. 6 - describe trace analysis and explain its role in...Ch. 6 - describe waves in terms of frequency, wavelength,...Ch. 6 - Prob. 3COCh. 6 - relate the frequency, wavelength, and amplitude of...Ch. 6 - describe the photoelectric effect by stating what...Ch. 6 - Prob. 6COCh. 6 - • use Planck’s equation to calculate the energy of...Ch. 6 - Prob. 8COCh. 6 - Prob. 9COCh. 6 - • describe similarities and differences between...
Ch. 6 - Prob. 11COCh. 6 - Prob. 12COCh. 6 - • identify an orbital (as 1s, 3p, etc.) from its...Ch. 6 - • list the number of orbitals of each type (1s,...Ch. 6 - • sketch the shapes of s and p orbitals and...Ch. 6 - • rank various orbitals in terms of size and...Ch. 6 - Prob. 17COCh. 6 - Prob. 18COCh. 6 - Prob. 19COCh. 6 - Prob. 20COCh. 6 - Prob. 6.1PAECh. 6 - 6.2 Unlike XRF, AAS cannot be used for...Ch. 6 - Prob. 6.3PAECh. 6 - Prob. 6.4PAECh. 6 - Prob. 6.5PAECh. 6 - Prob. 6.6PAECh. 6 - 6.7 Arrange the following regions of the...Ch. 6 - 6.8 Calculate the wavelength in meters, of...Ch. 6 - 6.9 If a string of decorative lights includes...Ch. 6 - 6.10 Define the term refraction.Ch. 6 - 6.11 Define the term photon.Ch. 6 - 6.12 Find the energy of a photon with each of the...Ch. 6 - 6.13 Place these types of radiation in order of...Ch. 6 - 6.14 For photon with the following energies,...Ch. 6 - Prob. 6.15PAECh. 6 - 6.16 Various optical disk drives rely on laser...Ch. 6 - 6.17 The laser in most supermarket barcode...Ch. 6 - 6.18 Assume that a microwave oven operates at a...Ch. 6 - 6.19 Fill in the blanks below to complete a...Ch. 6 - 6.20 When light with a wavelength of 58.5 nm...Ch. 6 - 6.21 The electron binding energy fur copper metal...Ch. 6 - Prob. 6.22PAECh. 6 - 6.23 Describe how the Bohr model of the atom...Ch. 6 - 6.24 According to the Bohr model of the atom, what...Ch. 6 - 6.25 Define the term ground state.Ch. 6 - 6.26 The figure below depicts the first four...Ch. 6 - 6.27 Refer w the data and energy-Ievel diagram...Ch. 6 - 6.28 A neon atom cmi light at many wavelengths,...Ch. 6 - 6.29 A mercury atom emits light at many...Ch. 6 - 6.30 How did the observation of electron...Ch. 6 - 6.31 Why do we use a wave function to describe...Ch. 6 - 6.32 What are the mathematical origins of quantum...Ch. 6 - Prob. 6.33PAECh. 6 - 6.34 Which of the following represent valid sets...Ch. 6 - 6.35 A particular orbital has n = 4 and l = 2....Ch. 6 - 6.36 Why are there no 2d orbitals?Ch. 6 - 6.34 What is the maximum number of electrons in an...Ch. 6 - 6.38 How many orbitals correspond to each of the...Ch. 6 - Prob. 6.39PAECh. 6 - 6.40 Referring to Figure 6.15, draw a 4p orbitals,...Ch. 6 - 6.41 Consider a 3d orbital. (a) What are the...Ch. 6 - Prob. 6.42PAECh. 6 - 6.43 Define the term spin paired.Ch. 6 - 6.44 On what does the Pauli exclusion principle...Ch. 6 - Prob. 6.45PAECh. 6 - Prob. 6.46PAECh. 6 - 6.47 Depict two ways to place electrons in the 2p...Ch. 6 - 6.48 Write the ground state electron configuration...Ch. 6 - 6.49 Which of these electron configurations are...Ch. 6 - 6.50 From the list of atoms and ions given,...Ch. 6 - Prob. 6.51PAECh. 6 - Prob. 6.52PAECh. 6 - Prob. 6.53PAECh. 6 - Prob. 6.54PAECh. 6 - 6.55 Explain why the s block of the periodic table...Ch. 6 - Prob. 6.56PAECh. 6 - Prob. 6.57PAECh. 6 - Prob. 6.58PAECh. 6 - Prob. 6.59PAECh. 6 - 6.60 Use the electron configurations of the alkali...Ch. 6 - 6.61 Using only a periodic table as a guide,...Ch. 6 - 6.62 Define the term ionization energy....Ch. 6 - 6.63 At which ionization for chlorine would you...Ch. 6 - 6.64 Arrange the following atoms in order of...Ch. 6 - Prob. 6.65PAECh. 6 - 6.66 Which element would you expect to have the...Ch. 6 - Prob. 6.67PAECh. 6 - 6.68 Indicate which species in each pair has the...Ch. 6 - 6.69 Compare the elements Na, B, Al, and C with...Ch. 6 - 6.70 Rank the following in order of decreasing...Ch. 6 - 6.71 Several excited states of the neon atom are...Ch. 6 - 6.72 LED bulbs offer a fairly new lighting...Ch. 6 - 6.73 How much energy could be saved each year by...Ch. 6 - Prob. 6.74PAECh. 6 - Prob. 6.75PAECh. 6 - Prob. 6.76PAECh. 6 - Prob. 6.77PAECh. 6 - Prob. 6.78PAECh. 6 - 6.79 How does the charge of electrons provide some...Ch. 6 - 6.80 Describe how valence electron configurations...Ch. 6 - 6.81 Why is there no element to the immediate...Ch. 6 - 6.82 A particular element has the following values...Ch. 6 - 6.83 The graph below shows the first three...Ch. 6 - 6.84 Which graph correctly depicts the first...Ch. 6 - 6.85 The visible lines in the hydrogen atom...Ch. 6 - 6.86 An excited He+ ion returns to the ground...Ch. 6 - Prob. 6.87PAECh. 6 - Prob. 6.88PAECh. 6 - Prob. 6.89PAECh. 6 - Prob. 6.90PAECh. 6 - 6.91 What is the only noble gas that does not have...Ch. 6 - 6.92 The photoelectric effect can he used to...Ch. 6 - 6.93 A mercury atom is initially in its lowest...Ch. 6 - Prob. 6.94PAECh. 6 - 6.95 A metallic sample is known to be barium,...Ch. 6 - 6.96 When a helium atom absorbs light at 58.44 nm,...Ch. 6 - 6.97 Arrange the members of each of the following...Ch. 6 - 6.98 Arrange the following sets of anions in order...Ch. 6 - 6.99 The photoelectric effect can he used in...Ch. 6 - 6.100 Some spacecraft use ion propulsion engines....Ch. 6 - 6.101 Laser welding is a technique in which a...Ch. 6 - Prob. 6.102PAECh. 6 - 6.103 Atomic absorption spectroscopy is based on...Ch. 6 - 6.104 The red color in fireworks is the result of...Ch. 6 - 6.105 When we say that the existence of atomic...Ch. 6 - 6.106 When Bohr devised his model for the atom,...Ch. 6 - Prob. 6.107PAE
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 6.84 Which graph correctly depicts the first ionization energy of three elements in groups 14 (dashed line) and 17 (solid line)? Explain the reasoning you used to make your choice.arrow_forwardRank the following elements according to their ionization energy. element polonium thallium krypton selenium ionization energy (Choose one) (Choose one) (Choose one) (Choose one) X Sarrow_forwardRank the following elements according to their ionization energy. element bromine arsenic bismuth thallium ionization energy (Choose one) (Choose one) (Choose one) (Choose one) x Śarrow_forward
- Rank the following elements according to their ionization energy. element ionization energy beryllium potassium 1 (highest) magnesium 4 (lowest) cesium 2arrow_forwardThe following data shows the successive ionization energies (in kJ/mol) for an element: IE1: 856 IE2: 2145 IE3: 3592 IE4: 23,721 IE5: 31,222 The element has type your answer... valence electrons (enter an integer in the box).arrow_forwardWhich of the following elements have a tendency to lose electrons? O Na, Ca, Al OCN, O O F, CI, Brarrow_forward
- Question 6 Which statement is true about ionization energies? O lonization energies for main group metals decrease when moving left to right in the same period on the periodic table. O lonization energies for main group metals decrease when moving down in the same group on the periodic table. O It is the amount of energy required to add an electron to an atom O It is the amount of energy released when removing a mole of electrons from a mole of atoms.arrow_forwardRank the folowing elements in order of increascing first ionization energy, smallest being closest to the top and largest at the bottom. = chlorine = magnesium = sodium = potassium = fluorinearrow_forwardWhat is the ionic charge of these elements? Ca, K, Al, Mg, S, O, P, Farrow_forward
- 8)arrow_forwardThe energy needed to remove an electron is called ionization energy. Which of the statements are true of ionization energy and its trends found in the periodic table? increases going down a column (top to bottom) decreases going down a column (top to bottom) increases going across a row (left to right) decreases going across a row (left to right)arrow_forwardRank the following elements according to their ionization energy. element magnesium sodium beryllium cesium ionization energy (Choose one) (Choose one) (Choose one) (Choose one) X 5arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Quantum Numbers, Atomic Orbitals, and Electron Configurations; Author: Professor Dave Explains;https://www.youtube.com/watch?v=Aoi4j8es4gQ;License: Standard YouTube License, CC-BY
QUANTUM MECHANICAL MODEL/Atomic Structure-21E; Author: H to O Chemistry;https://www.youtube.com/watch?v=mYHNUy5hPQE;License: Standard YouTube License, CC-BY