
(a)
Interpretation:
Calculate values of
Concept Introduction:
The Gibbs free energy is calculated as:
(a)
Answer to Problem 6.46P
Explanation of Solution
Given information:
It is given that pressure is
From steam tables of saturated steam in Appendix E, table E.1
At pressure
Since, pressure
From linear interpolation, if
Temperature corresponding to
Enthalpy of saturated liquid and entropy of saturated liquid and vapor is
And,
Entropy of saturated liquid and entropy of saturated liquid and vapor is
And,
So,
And
Both values of
(b)
Interpretation:
Calculate values for
Concept Introduction:
The change in enthalpy and entropy in ideal gas is defined as:
And
(b)
Answer to Problem 6.46P
Explanation of Solution
From subpart (a), values of enthalpies of saturated vapor and liquid as well as values of entropies of saturated vapor and liquid are:
And temperature is:
So,
And
Both values of
(c)
Interpretation:
Calculate values for
Concept Introduction:
The residual properties
The residual properties
The residual properties
(c)
Answer to Problem 6.46P
Explanation of Solution
At
So,
Now, for the hypothetical ideal gas values of volume, enthalpy and entropy at same temperature and pressure, it cannot find using steam table because steam tables only give values of real gases not ideal gases. However, we can make an approximation of low pressure in real gases at the same temperature to convert real gas into ideal one. So, we are considering low pressure or
Since,
Hence from linear interpolation, at
But we want values of volume, enthalpy and entropy of ideal gas at
And
Enthalpy of ideal gas is defined as
Which is not the function of pressure, so enthalpy of ideal gas at
Now,
For entropy we know that
Since temperature s constant so first term at right hand side will be zero and hence,
So,
Residual properties are:
And
And
Now calculations of residual properties of volume, enthalpy and entropy for saturated vapor from generalized correlations are given below,
....(1a)
Where,
....(1b)
....(2a)
Where,
....(2b)
And
....(3a)
Where
....(3b)
Properties of pure species of steam are given in Table B.1 Appendix B as water,
So,
And
For differentiative terms in equation (2b) and (3b),
And,
For residual volume calculations, From equation (1b)
So, from equation (1a)
For residual enthalpy calculations, From equation (2b)
So, from equation (2a)
For residual entropy calculations, from equation (3b)
So, from equation (3a)
Results do not agree but approximately they do agree.
(d)
Interpretation:
Calculate values for
Concept Introduction:
First draw a graph between
The Clapeyron equation is:
(d)
Answer to Problem 6.46P
Explanation of Solution
From saturated steam table in Appendix E, table E.2
Now, draw a graph between
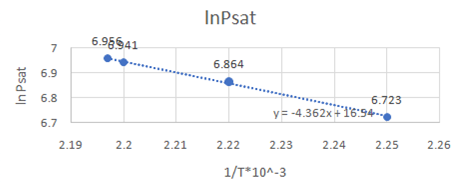
Slope of the graph from equation of graph is
Or,
So,
Also,
Volume of saturated liquid and entropy of saturated liquid and vapor at pressure
Since, pressure
And,
So,
Now from Clapeyron equation,
The value of
So, the results approximately match with each other.
Want to see more full solutions like this?
Chapter 6 Solutions
Loose Leaf For Introduction To Chemical Engineering Thermodynamics
- For spherical sand particles with Dp = 0.03 and ρparticles = 150 lbm / ft3 estimate the minimum fluidizing velocity for air and for water. Assume ε = 0.3. In the case of the water we must rederive Eq. 11.42, taking into account the buoyant force on the particles. Below are the provide answers. Please show all work to get to the correct answers.arrow_forwardPlease show all workarrow_forward2. A moving bed adsorption column needs to be designed to separate hydrophobic proteins from a fermentation broth. The following adsorption equilibrium data was observed in preliminary isotherm studies. The resin used was activated carbon with a porosity of 0.2. The overall mass transfer coefficient was determined to be 10 h¹, and the ratio of volumetric flow rate of broth to resin is 10. Determine the diameter of the column if the column height is limited to 2.5 m (indoor operation) with a flow rate of 20 m³/h, influent concentration of 7 g/L, and effluent concentration of 0.1 g/L. qi (mg/kg) Ci (g/L) 0.1 4.7 7.5 0.25 10.6 0.5 15.0 1.0 23.7 2.5 33.5 5.0 41.1 7.5arrow_forward
- 3. You are given a mixture of four proteins, whose properties are listed in the table below. Propose a process to purify each protein so that you end up with four solutions of pure protein. What resin would you use to bind the protein(s)? What changes to the buffer would you make to desorb the protein(s)? Contains an N-terminal His6-tag. Two 50 kDa subunits contain a non-heme Fe2+ in the active site. Protein Size (kDa) pl Specific Properties A 100 6.0 B 40 7.7 C 240 8.3 Ꭰ 225 5.5 Contains FAD redox center and an NADH binding domain. Composed of six 40-kDa subunits, each of which contains a [2Fe-2S] cluster. Composed of three subunits: 100 kDa structural subunit, 75 kDa subunit with a molybdopterin center, and 50 kDa subunit with FAD and an NADH binding domain.arrow_forwardb) Explain the key features of the Langmuir adsorption model - Drawing a diagram with empty and occupied sites. Show how new molecules would adsorb. drawing the diagram, showing free and empty sites, and their number (to use for next section) - Define the capacity and binding affinity parameters in terms of things shown on the diagram Defining the capacity and binding affinity parameters in terms of bound, free sites, and free molecules - Plot what would be a typical breakthrough curve and give an explanation approximately when breakthrough would occur plotting a typical sigmoidal breakthrough curve and saying it would certainly occur by the time capacity is used, but also could be much earlier if the affinity is lowarrow_forwardWater at 20°C flows at a steady average velocity of 5.25 m/s through a smooth pipe of diameter 5.08 cm. The flow is fully developed through the entire section of pipe. The total pipe length is 10.56 m, and there are two 90' elbows. Determine the friction coefficient and the head loss due to friction per meter length of the pipe. Control volume Prepared by Engr. Kirsten Gaarrow_forward
- Problem 2. For an irreversible liquid phase reaction A -> B, the reaction rate is of the first order with respect to the reactant concentration C_A. this reaction is performed in a cascade of two identical CSTRs at 100 degrees Celsius. (same reactor size and isothermal). The inlet concentration of A of the first CSTR is 2mol/L. The outlet concentration of A of the 2nd CSTR is 0.5 mol/L. the inlet flow rate of the 1st reactor is 100 L/h. and the feed temperature is 20 degrees Celsius. The average heat capacity of the reactant/product/solvent mixture is a constant: 2J/g*K, the density of the mixture is a constant: 1 kg/L. The heat of reaction is 50 kJ/mol (exothermic). The reaction rate constant at 100 degrees Celsius is 0.5/h. (a) Determine the outlet concentration of A of the first CSTR (b) What is the heat transfer requirement for the first CSTR? (c) if this reaction is performed in a plug-flow reactor, what is the size of plug-flow reactor required for achieving the same conversion…arrow_forwardThe energy release (Q_g) and energy loss (Q_r) curves of an irreversible oxidation reaction are shown below. Q_r curves can be shifted by adjusting the feed temperature. Q,& QE E Qg (a) Are these points of intersection between energy release and energy loss curves stable operating conditions? Point of Intersection A Stable or Unstable B A D T (b) Which point represents the ignition condition? B с D E F Garrow_forwardProblem 1. For an irreversible liquid phase reaction 2A -> B, the reaction rate is of the 2nd order with respect to the reactant concentration CA. The concentration-dependent reaction rate is plotted below. This reaction is performed in a cascade of two identical CSTRS (same reactor size and temperature). The inlet concentration of A of the 1st CSTR is 2 mol/L. The outlet concentration of A of the 2nd CSTR is 1 mol/L. The inlet flow rate of the 1st reactor is 100 L/h. Please use the graphical method to determine the outlet concentration of A of the first CSTR and the size of each CSTR. Please briefly show the procedure for reactor size calculation. (-4-7) 15225050 45 40 35 30 0 0.5 11.761.5 C₂ Q C (mol.L¹) Co 20 2.5arrow_forward
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





