
Concept explainers
(a)
Interpretation:
All curved arrows that show the entire transformation of compound 1 into 6 have to be drawn and entire sequance of arrow pushing patterns should be identified.
Concept Introduction:
Mechanism of the reaction is the step-by-step description of the process by which reactants are changed into products.
There are only four characteristic patterns, and all ionic mechanisms are simply combinations of these four steps, and they are,
- (1) Nucleophilic attack
When we identify a nucleophilic site and an electrophilic site, the arrow in the mechanism step shows the nucleophile attacking.
- (2) Proton transfer
- (3) Loss of leaving group
- (4) Rearrangements
Rearrangements will always occur when an alkyl group or hydrogen can shift to form a more stable carbocation. There are mainly two types of rearrangement shifts and they are,
Curved arrows show the bonds that are formed and the bonds that are broken in a reaction.
Curved arrows used to understand a reaction mechanism.
Curved arrows are drawn to show how the electrons move as new covalent bonds are formed existing covalent bonds are broken.
Each arrow represents the simultaneous movement of two electrons from a nucleophile towards an electrophile.
Nucleophile: It is negatively charged species which seeks for positive charge and hence donate pair of electrons to positively charged species (electrophiles) which results in the formation of
(b)
Interpretation:
Nucleophilic and eletrophilic centers in compound 4 has to be identified and use resonance structures to justify why the latter is electron poor.
Concept Introduction:
Electrophile: It is positively charged species which seeks for negative charge and hence accepts pair of electrons from negatively charged species (Nucleophiles) which results in the formation of chemical bond.
Electrophiles have a specific atom or region of the molecule which is electron deficient. This region is called the electrophilic center.
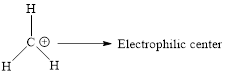
Nucleophile: It is negatively charged species which seeks for positive charge and hence donate pair of electrons to positively charged species (electrophiles) which results in the formation of chemical bond.
Nucleophiles have a specific atom or region of the molecule which is electron excess. This region is called the electrophilic center.
Resonance is an electron displacement effect for stabilizing a molecule through delocalization of bonding electrons in the pi orbital.
Delocalized electrons stabilize a compound. The extra stability gains from having delocalized electrons are called resonance stabilization or resonance energy.
(c)
Interpretation:
New chiral center which is formed in the conversion of 4 into 5 has to be identified and its configuration has to assigned. It should be explained that why the other possible configuration is not observed.
Concept Introduction:
The stereoisomerism is the arrangement of atoms in molecules whose connectivity remains the same but their arrangement in different in each isomer.
Chiral centre: A chiral centre is defined as the tetrahedral carbon atom in an organic molecule that is connected to four non-identical groups/substituents. It is sometimes known as a stereo genic centre.
Chirality: The geometric property of molecules where the structure of the molecule and its mirror image are not superimposable is known as chirality. Chiral molecules are optically active and they can rotate the plane polarized light.
R and S nomenclature: it is used to assign the molecule using CIP (Cahn-Ingold-Prelog) rules.
The CIP rules are as follows:
Select the chiral carbon and assign the numbers according to the decreasing
If the numbering follows clockwise direction then the molecule is termed as R and if it follows anti-clockwise direction then molecule is termed as S.
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
Organic Chemistry
- in the kinetics experiment, what were the values calculated? Select all that apply.a) equilibrium constantb) pHc) order of reactiond) rate contstantarrow_forwardtrue or false, given that a 20.00 mL sample of NaOH took 24.15 mL of 0.141 M HCI to reach the endpoint in a titration, the concentration of the NaOH is 1.17 M.arrow_forwardin the bromothymol blue experiment, pKa was measured. A closely related compound has a Ka of 2.10 x 10-5. What is the pKa?a) 7.1b) 4.7c) 2.0arrow_forward
- calculate the equilibrium concentration of H2 given that K= 0.017 at a constant temperature for this reaction. The inital concentration of HBr is 0.050 M.2HBr(g) ↔ H2(g) + Br2(g)a) 4.48 x 10-2 M b) 5.17 x 10-3 Mc) 1.03 x 10-2 Md) 1.70 x 10-2 Marrow_forwardtrue or falsegiven these two equilibria with their equilibrium constants:H2(g) + CI2(l) ↔ 2HCI(g) K= 0.006 CI2(l) ↔ CI2(g) K= 0.30The equilibrium contstant for the following reaction is 1.8H2(g) + CI2 ↔ 2HCI(g)arrow_forwardI2(g) + CI2(g) ↔ 2ICIK for this reaction is 81.9. Find the equilibrium concentration of I2 if the inital concentration of I2 and CI2 are 0.010 Marrow_forward
- true or false,the equilibrium constant for this reaction is 0.50.PCI5(g) ↔ PCI3(g) + CI2(g)Based on the above, the equilibrium constant for the following reaction is 0.25.2PCI5(g) ↔. 2PCI3(g) + 2CI2(g)arrow_forwardtrue or false, using the following equilibrium, if carbon dioxide is added the equilibrium will shift toward the productsC(s) + CO2(g) ↔ 2CO(g)arrow_forward2S2O2/3- (aq) + I2 (aq) ---> S4O2/6- (aq) +2I- (aq) Experiment I2 (M) S2O3- (M) Initital Rate (M/s) 1 0.01 0.01 0.0004 2 0.01 0.02 0.0004 3 0.02 0.01 0.0008 Calculate the overall order for this reaction using the table data a) 3b) 0c) 2d) 1arrow_forward
- the decomposition of N2O5 is the first order with a half-life of 1.98 minutes. If the inital concentration of N2O5 is 0.200 M, what is the concentration after 6 minutes?a) 0.612 Mb) 0.035 Mc) 0.024 Md) 0.100 Marrow_forward20.00 mL of 0.150 M HCI is titrated with 0.075 M NaOH. What volume of NaOH is needed?a) 50 mLb) 20 mLc) 40 mLd) 26.66 mLarrow_forward20.00 mL of 0.150 M NaOH is titrated with 37.75 mL of HCI. What is the molarity of the HCI?a) 0.150 Mb) 0.079 Mc) 0.025 Md) 0.050 Marrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





