
Chemistry: Structure and Properties (2nd Edition)
2nd Edition
ISBN: 9780134293936
Author: Nivaldo J. Tro
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 6, Problem 2SAQ
Determine the hybridization about C in H2CO.
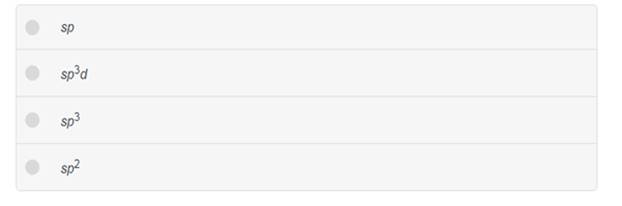
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
bre
The reaction sequence shown in Scheme 5 demonstrates the synthesis of a
substituted benzene derivative Q.
wolsd works 2
NH2
NaNO2, HCI
(apexe) 13× (1
HNO3, H2SO4
C6H5CIN2
0°C
HOTE
CHINO₂
N
O
*O₂H (
PO
Q
Я
Scheme 5
2 bag abouoqmics to sounde odi WEIC
(i)
Draw the structure of intermediate O.
[2 marks]
to noitsmot od: tot meinedogm, noit so oft listsb ni zaupaib bas wa
(ii) Draw the mechanism for the transformation of aniline N to intermediate O.
Spoilage
(b)
[6 marks]
(iii) Identify the reagent X used to convert compound O to the iodinated compound
[tom E
P.
vueimado oilovonsa ni moitos nolisbnolov ayd toes ai tedw nisiqx
(iv) Identify the possible structures of compound Q.
[2 marks]
[2 marks]
[shom 2]
(v)
bus noires goiribbeolovo xnivollot adj to subora sidab
Draw the mechanism for the transformation of intermediate P to compound Q.
[5 marks]
vi
(vi) Account for the regiochemical outcome observed in the reaction forming
compound Q.
[3 marks]
PROBLEM 4 Solved
Show how 1-butanol can be converted into the following compounds:
a.
PROBLEM 5+
b.
d.
-C=
N
Which alkene is the major product of this dehydration?
OH
H2SO4
heat
Chapter 6 Solutions
Chemistry: Structure and Properties (2nd Edition)
Ch. 6 - Prob. 1ECh. 6 - What is a chemical bond according to valence bond...Ch. 6 - In valence bond theory, what determines the...Ch. 6 - In valence bond theory, the interaction energy...Ch. 6 - What is hybridization? Why is hybridization...Ch. 6 - How does hybridization of the atomic orbitals in...Ch. 6 - How is the number of hybrid orbitals related to...Ch. 6 - Sketch each hybrid orbital sp sp2 sp3 sp3d sp3d2Ch. 6 - Prob. 9ECh. 6 - Name the hybridization scheme that corresponds to...
Ch. 6 - What is a chemical bond according to molecular...Ch. 6 - Explain the difference between hybrid atomic...Ch. 6 - What is a bonding molecular orbital?Ch. 6 - Prob. 14ECh. 6 - What is the role of wave interference in...Ch. 6 - Prob. 16ECh. 6 - Prob. 17ECh. 6 - Prob. 18ECh. 6 - Prob. 19ECh. 6 - Prob. 20ECh. 6 - Prob. 21ECh. 6 - When applying molecular orbital theory to...Ch. 6 - In molecular orbital theory, what is a nonbonding...Ch. 6 - Write a short paragraph describing chemical...Ch. 6 - The valence electron configurations of several...Ch. 6 - The valence electron configurations of several...Ch. 6 - Draw orbital diagrams (boxes with arrows in them)...Ch. 6 - Draw orbital diagrams (boxes with arrows in them)...Ch. 6 - Prob. 29ECh. 6 - Draw orbital diagrams (boxes with arrows in them)...Ch. 6 - Which hybridization scheme allows the formation of...Ch. 6 - Which hybridization scheme allows the central atom...Ch. 6 - Write a hybridization and bonding scheme for each...Ch. 6 - Write a hybridization and bonding scheme for each...Ch. 6 - Write a hybridization and bonding scheme for each...Ch. 6 - Write a hybridization and bonding scheme for each...Ch. 6 - Write a hybridization and bonding scheme for each...Ch. 6 - Write a hybridization and bonding scheme for each...Ch. 6 - Consider the structure of the amino acid alanine...Ch. 6 - Consider the structure of the amino acid aspartic...Ch. 6 - Sketch the bonding molecular orbital that results...Ch. 6 - Sketch the antibonding molecular orbital that...Ch. 6 - Draw an MO energy diagram and predict the bond...Ch. 6 - Draw an MO energy diagram and predict the bond...Ch. 6 - Sketch the bonding and antibonding molecular...Ch. 6 - Sketch the bonding and antibonding molecular...Ch. 6 - Using the molecular orbital energy ordenng for...Ch. 6 - Using the molecular orbital energy ordering for...Ch. 6 - Apply molecular orbital theory to predict if each...Ch. 6 - Apply molecular orbital theory to predict if each...Ch. 6 - According to MO theory, which molecule or ion has...Ch. 6 - According to MO theory, which molecule or ion has...Ch. 6 - Draw an MO energy diagram for CO. (Use the energy...Ch. 6 - Draw an MO energy diagram for HCI. Predict the...Ch. 6 - For each compound, draw the Lewis structure,...Ch. 6 - For each compound, draw the Lewis structure,...Ch. 6 - Amino acids are biological compounds that link...Ch. 6 - The genetic code is based on four different bases...Ch. 6 - The structure of caffeine, present in coffee and...Ch. 6 - The structure of acetylsalicylic acid (aspirin) is...Ch. 6 - Draw a molecular orbital energy diagram for CIF....Ch. 6 - Draw Lewis structures and MO diagrams for CN+, CN,...Ch. 6 - Bromine can form compounds or ions with any number...Ch. 6 - The compound C3H4 has two double bonds. Describe...Ch. 6 - How many hybrid orbitals do we use to describe...Ch. 6 - Prob. 66ECh. 6 - In VSEPR theory, which uses the Lewis model to...Ch. 6 - The resuts of a molecular orbital calculation for...Ch. 6 - Prob. 69ECh. 6 - cis-2-Butene isomerizes (changes its structure) to...Ch. 6 - The ion CH5 + can form under very special...Ch. 6 - Neither the VSEPR model nor the hybridization...Ch. 6 - Prob. 73ECh. 6 - The most stable forms of the nonmetals in groups...Ch. 6 - Consider the bond energies of three iodine...Ch. 6 - How many atomic orbitals form a set of sp3hybrid...Ch. 6 - Have each group member pick one of these...Ch. 6 - Divide your group into two subgroups. Have one...Ch. 6 - A molecular orbital calculation for Hi results in...Ch. 6 - Determine the hybridization about 0 in CH3OH.Ch. 6 - Determine the hybridization about C in H2CO.Ch. 6 - According to the valance bond theory, which kind...Ch. 6 - Use molecular orbital theory to determine the bond...Ch. 6 - Use molecular orbital theory to predict which...Ch. 6 - Use molecular orbital theory to determine which...Ch. 6 - Which hybridization scheme occurs about nitrogen...Ch. 6 - Prob. 8SAQCh. 6 - Prob. 9SAQCh. 6 - Prob. 10SAQCh. 6 - Which type of orbitals overlap to form the sigma...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please correct answer and don't used hand raiting and don't used Ai solutionarrow_forwardPlease correct answer and don't used hand raitingarrow_forwardThe vibrational contribution isa) temperature independent for internal energy and heat capacityb) temperature dependent for internal energy and heat capacityc) temperature independent for heat capacityd) temperature independent for internal energyarrow_forward
- Quantum mechanics. Explain the basis of approximating the summation to an integral in translational motion.arrow_forwardQuantum mechanics. In translational motion, the summation is replaced by an integral when evaluating the partition function. This is correct becausea) the spacing of the translational energy levels is very small compared to the product kTb) the spacing of the translational energy levels is comparable to the product kTc) the spacing of the translational energy levels is very large compared to the product kTarrow_forwardDon't used Ai solutionarrow_forward
- Please correct answer and don't used hand raiting don't used Ai solutionarrow_forwardIf the viscosity of hydrogen gas (at 0oC and 1 atm) is 8.83x10-5 P. If we assume that the molecular sizes are equal, calculate the viscosity of a gas composed of deuterium.arrow_forwardIf the viscosity of hydrogen gas (at 0oC and 1 atm) is 8.83x10-5 P. If we assume that the molecular sizes are equal, calculate the viscosity of a gas composed of deuterium.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning


Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning
Types of bonds; Author: Edspira;https://www.youtube.com/watch?v=Jj0V01Arebk;License: Standard YouTube License, CC-BY