
Student's Study Guide and Solutions Manual for Organic Chemistry
8th Edition
ISBN: 9780134066585
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 5.12, Problem 36P
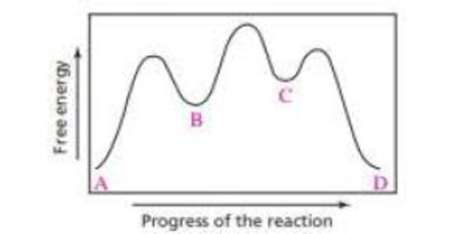
a. Which step in the reaction coordinate diagram shown here has the, greatest free energy of activation in the forward direction?
b. Is the first-formed intermediate more apt to revert to reactants or go on to form products?
c. Which step is the rate-determining step of the reaction?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
3.
a.
Use the MS to propose at least two possible molecular formulas.
For an unknown compound:
101.
27.0
29.0
41.0
50.0
52.0
55.0
57.0
100
57.5
58.0
58.5
62.0
63.0
64.0
65.0
74.0
40
75.0
76.0
20
20
40
60
80
100
120
140
160
180
200 220
m/z
99.5
68564810898409581251883040
115.0
116.0
77404799
17417M
117.0
12.9
118.0
33.5
119.0
36
133 0
1.2
157.0
2.1
159.0
16
169.0
219
170.0
17
171.0
21.6
172.0
17
181.0
1.3
183.0
197.0
100.0
198.0
200.
784
Relative Intensity
2
2
8
ō (ppm)
6
2
Solve the structure and assign each of the following spectra (IR and C-NMR)
1.
For an unknown compound with a molecular formula of C8H100:
a.
What is the DU? (show your work)
b.
Solve the structure and assign each of the following spectra.
8
6
2
ō (ppm)
4
2
0
200
150
100
50
ō (ppm)
LOD
D
4000
3000
2000
1500
1000
500
HAVENUMBERI -11
Chapter 5 Solutions
Student's Study Guide and Solutions Manual for Organic Chemistry
Ch. 5.1 - What is the molecular formula for each of the...Ch. 5.1 - Prob. 4PCh. 5.1 - Determine the degree of unsaturation and then draw...Ch. 5.1 - Prob. 6PCh. 5.2 - What is each compounds systematic name?Ch. 5.2 - Prob. 8PCh. 5.2 - Draw the structure for each of the following: a....Ch. 5.3 - How many carbons are in the planar double-bond...Ch. 5.3 - Prob. 12PCh. 5.5 - Prob. 13P
Ch. 5.5 - Prob. 14PCh. 5.5 - Prob. 16PCh. 5.5 - Prob. 17PCh. 5.6 - a. Which of the monosubstituted cyclohexanes in...Ch. 5.6 - a. Calculate the percentage of isopropylcylohexane...Ch. 5.6 - a. for which reaction in each set will S be more...Ch. 5.6 - a. For a reaction with H = 12 kcal/ mol and S =...Ch. 5.8 - Prob. 23PCh. 5.9 - Prob. 24PCh. 5.9 - How many different alkenes can be hydrogenated to...Ch. 5.9 - The same alkane is obtained from the catalytic...Ch. 5.9 - Prob. 27PCh. 5.9 - Rank the following compounds from most stable to...Ch. 5.10 - Prob. 29PCh. 5.10 - Prob. 30PCh. 5.11 - The rate constant for a reaction can be increased...Ch. 5.11 - Prob. 33PCh. 5.11 - a. Which reaction has a greater equilibrium...Ch. 5.12 - Draw a reaction coordinate diagram for a two-step...Ch. 5.12 - a. Which step in the reaction coordinate diagram...Ch. 5.12 - Draw a reaction coordinate diagram for the...Ch. 5.13 - Prob. 38PCh. 5 - What is each compounds systematic name?Ch. 5 - Prob. 40PCh. 5 - Draw the structure of a hydrocarbon that has six...Ch. 5 - Draw the condensed structure for each of the...Ch. 5 - Prob. 43PCh. 5 - Prob. 44PCh. 5 - Prob. 45PCh. 5 - Name the following:Ch. 5 - Prob. 47PCh. 5 - Prob. 48PCh. 5 - Prob. 49PCh. 5 - In a reaction in which reactant A is in...Ch. 5 - Which bond is stronger? Briefly explain why.Ch. 5 - Prob. 52PCh. 5 - Prob. 53PCh. 5 - By following the curved red arrows, draw the...Ch. 5 - Prob. 55PCh. 5 - Prob. 56PCh. 5 - Draw structures for the following: a....Ch. 5 - Prob. 58PCh. 5 - a. Which of the following reactions has the larger...Ch. 5 - Prob. 60PCh. 5 - a. What is the equilibrium constant for a reaction...Ch. 5 - Prob. 62PCh. 5 - Prob. 63PCh. 5 - Given that the free energy of the twist-boat...Ch. 5 - Prob. 65PCh. 5 - Prob. 1PCh. 5 - Prob. 2PCh. 5 - Prob. 3PCh. 5 - Prob. 4PCh. 5 - Prob. 5PCh. 5 - Prob. 6PCh. 5 - Draw curved arrows to show the movement of the...Ch. 5 - Prob. 8PCh. 5 - Prob. 9PCh. 5 - Prob. 10P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 16. The proton NMR spectral information shown in this problem is for a compound with formula CioH,N. Expansions are shown for the region from 8.7 to 7.0 ppm. The normal carbon-13 spec- tral results, including DEPT-135 and DEPT-90 results, are tabulated: 7 J Normal Carbon DEPT-135 DEPT-90 19 ppm Positive No peak 122 Positive Positive cus и 124 Positive Positive 126 Positive Positive 128 No peak No peak 4° 129 Positive Positive 130 Positive Positive (144 No peak No peak 148 No peak No peak 150 Positive Positive してしarrow_forward3. Propose a synthesis for the following transformation. Do not draw an arrow-pushing mechanism below, but make sure to draw the product of each proposed step (3 points). + En CN CNarrow_forwardShow work..don't give Ai generated solution...arrow_forward
- Label the spectrum with spectroscopyarrow_forwardQ1: Draw the most stable and the least stable Newman projections about the C2-C3 bond for each of the following isomers (A-C). Are the barriers to rotation identical for enantiomers A and B? How about the diastereomers (A versus C or B versus C)? enantiomers H Br H Br (S) CH3 H3C (S) (R) CH3 H3C H Br A Br H C H Br H3C (R) B (R)CH3 H Br H Br H3C (R) (S) CH3 Br H D identicalarrow_forwardLabel the spectrumarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Physical Chemistry
Chemistry
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Wadsworth Cengage Learning,
How to Design a Total Synthesis; Author: Chemistry Unleashed;https://www.youtube.com/watch?v=9jRfAJJO7mM;License: Standard YouTube License, CC-BY