
Concept explainers
(a)
Interpretation: The elements with the given electronic configuration should be identified as a metal, non-metal or metalloid.
Concept Introduction: The elements which can donate electrons to get the stable electronic configuration are metal, elements that can accept electrons are non-metal. The elements with characteristics of both metals and non-metals are metalloids.
(b)
Interpretation: The element with the smallest atomic size should be identified.
Concept Introduction: According to the periodic trends, the atomic size of the element increases on moving top to bottom in a group due to addition of one shell and decreases on moving left to right in a group due to increase in
(c)
Interpretation: The element with the highest ionization energy should be identified.
Concept Introduction: Ionization energy is defined as the amount of energy required to remove an electron from its outermost shell. According to periodic trends, it decreases on moving top to bottom in a group due to increase in size and it increases on moving left to right in a period due to decrease in size.
(d)
Interpretation: The element with the half-filled sublevel should be identified.
Concept Introduction: The relative energy of orbitals is represented as follows:
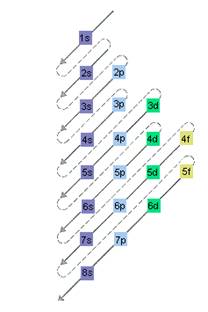
A s orbital can have maximum of 2 electrons, p orbital can have maximum of 6 electrons. Similarly, maximum electrons that a d and f orbital can have are 10 and 14 respectively.
Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
EP BASIC CHEMISTRY-STANDALONE ACCESS
- I'm confused can you help me for steps data analysis in AFMarrow_forwardHow many equivalents are in 15.0ml of 0.12 M BA(OH)2 solution? What volume of 0.085 M HNO3 is required to reach the endpoint when titration 15.0 ml of this solution?arrow_forwardWhy doesn't this undergo a 1,2-shift when it's in the carbocation stage of the reaction?arrow_forward
- 4. Redraw the following compounds from most reduced to most oxidized. If compounds have identical oxidation states, draw them under each other. OH میر محمد ملک OH OH .OH OH HS سلام پر من OH most reduced most oxidizedarrow_forwardPlease correct answer and don't used hand raitingarrow_forwardAfter 2-Bromo-3,3-dimethylbutane was reacted with KOC(CH3)3 a 1H NMR spectrum was obtained of the product. Propose a structure for the product of this reaction.arrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





