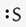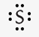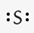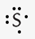Chemistry: Structure and Properties Custom Edition for Rutgers University General Chemistry
15th Edition
ISBN: 9781269935678
Author: Nivaldo J. Tro
Publisher: Pearson Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 5, Problem 8SAQ
What is the correct Lewis symbol for S?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Can u help me figure out the reaction mechanisms for these, idk where to even start
Hi, I need your help with the drawing, please. I have attached the question along with my lab instructions. Please use the reaction from the lab only, as we are not allowed to use outside sources. Thank you!
Hi, I need your help i dont know which one to draw please. I’ve attached the question along with my lab instructions. Please use the reaction from the lab only, as we are not allowed to use outside sources. Thank you!
Chapter 5 Solutions
Chemistry: Structure and Properties Custom Edition for Rutgers University General Chemistry
Ch. 5 - What is the empirical formula of the compound with...Ch. 5 - Which substance is an ionic compound? He N2O4 CCl4...Ch. 5 - Prob. 3SAQCh. 5 - Prob. 4SAQCh. 5 - Prob. 5SAQCh. 5 - Prob. 6SAQCh. 5 - Prob. 7SAQCh. 5 - What is the correct Lewis symbol for S?Ch. 5 - How many CH2Cl2 molecules are there in 25.0 g of...Ch. 5 - List the elements in the compound CF2Cl2 in order...
Ch. 5 - Determine the mass of potassium in 35.5 g of KBr....Ch. 5 - A compound is 52.14% C, 13.13% H, and 34.73% O by...Ch. 5 - A compound has the empirical formula CH2O and a...Ch. 5 - Combustion of 30.42 g of a compound containing...Ch. 5 - How do the properties of compounds compare to the...Ch. 5 - What is a chemical bond? Why do chemical bonds...Ch. 5 - Explain the difference between an ionic bond and a...Ch. 5 - List and describe the different ways to represent...Ch. 5 - What is the difference between an empirical...Ch. 5 - Prob. 6ECh. 5 - Prob. 7ECh. 5 - Prob. 8ECh. 5 - How can you use Lewis structures to determine the...Ch. 5 - What is lattice energy?Ch. 5 - Why is the formation of solid sodium chloride from...Ch. 5 - Prob. 12ECh. 5 - Prob. 13ECh. 5 - Prob. 14ECh. 5 - Prob. 15ECh. 5 - Prob. 16ECh. 5 - Prob. 17ECh. 5 - How does the Lewis model for covalent bonding...Ch. 5 - Explain howto nans molecular inorganic compounds.Ch. 5 - Prob. 20ECh. 5 - What is the formula mass for a compound? Why is it...Ch. 5 - Explain how the information in a chemical formula...Ch. 5 - What is mass percent composition? Why is it...Ch. 5 - Which kinds of conversion factors are inherent in...Ch. 5 - Which kind of chemical formula can be obtained...Ch. 5 - Prob. 26ECh. 5 - Prob. 27ECh. 5 - Prob. 28ECh. 5 - Prob. 29ECh. 5 - Prob. 30ECh. 5 - Prob. 31ECh. 5 - Determine the empirical formula for the compound...Ch. 5 - Determine the number of each type of atom in each...Ch. 5 - Determine the number of each type of atom in each...Ch. 5 - Write a chemical formula for each molecular model....Ch. 5 - Prob. 36ECh. 5 - Prob. 37ECh. 5 - Write an electron configuration for Ne. Then write...Ch. 5 - Prob. 39ECh. 5 - Write a Lewis symbol for each atom or ion. a. S2-...Ch. 5 - Prob. 41ECh. 5 - Write the Lewis symbols that represent the ions in...Ch. 5 - Prob. 43ECh. 5 - Prob. 44ECh. 5 - The lattice energy of CsF is -744 kJ/mol, whereas...Ch. 5 - Rubidium iodide has a lattice energy of-617...Ch. 5 - Prob. 47ECh. 5 - Prob. 48ECh. 5 - Prob. 49ECh. 5 - Prob. 50ECh. 5 - Prob. 51ECh. 5 - Prob. 52ECh. 5 - Prob. 53ECh. 5 - Prob. 54ECh. 5 - Prob. 55ECh. 5 - Prob. 56ECh. 5 - Prob. 57ECh. 5 - Prob. 58ECh. 5 - Prob. 59ECh. 5 - Prob. 60ECh. 5 - Use covalent Lewis structures to explain why each...Ch. 5 - Use covalent Lewis structures to explain why the...Ch. 5 - Prob. 63ECh. 5 - Prob. 64ECh. 5 - Prob. 65ECh. 5 - Prob. 66ECh. 5 - Name each compound. (Refer to the nomenclature...Ch. 5 - Prob. 68ECh. 5 - Prob. 69ECh. 5 - Prob. 70ECh. 5 - Calculate the formula mass for each compound. NO2...Ch. 5 - Prob. 72ECh. 5 - Calculate the number of moles in each sample 72.5...Ch. 5 - Calculate the mass of each sample 15.7 mol HNO3...Ch. 5 - Determine the number of moles (of molecules or...Ch. 5 - Determine the number of moles (of molecules or...Ch. 5 - How many molecules are in each sample? 6.5 g H2O...Ch. 5 - Prob. 78ECh. 5 - Calculate the mass (in g) of each sample. 5.94 x...Ch. 5 - Calculate the mass (in g) of each sample 4.5 x...Ch. 5 - A sugar crystal contains approximately 1.8 x 1017...Ch. 5 - A salt crystal has a mass of 0.12 mg. How many...Ch. 5 - Calculate the mass percent composition of carbon...Ch. 5 - Calculate the mass percent composition of nitrogen...Ch. 5 - Most fertilizers consist of nitrogen-containing...Ch. 5 - Iron in the earth is in the form of iron ore....Ch. 5 - Copper(ll) fluoride contains 37.42% F by mass....Ch. 5 - Silver chloride, often used in silver plating,...Ch. 5 - The iodide ion is a dietary mineral essential to...Ch. 5 - The American Dental Association recommends that an...Ch. 5 - Write a ratio showing the relationship between the...Ch. 5 - Write a ratio showing the relationship between the...Ch. 5 - Determine the number of moles of hydrogen atoms in...Ch. 5 - Determine the number of moles of oxygen atoms in...Ch. 5 - Calculate mass (in grams) of sodium in 8.5 g of...Ch. 5 - Calculate the mass (in kilograms) of chlorine in...Ch. 5 - A chemist decomposes samples of several compounds;...Ch. 5 - A chemist decomposes samples of several compounds;...Ch. 5 - Calculate the empirical formula for each stimulant...Ch. 5 - Calculate the empirical formula for each natural...Ch. 5 - The elemental mass percent composition of...Ch. 5 - The elemental mass percent composition of ascorbic...Ch. 5 - A 0.77-mg sample of nitrogen reacts with chlorine...Ch. 5 - A 45.2-mg sample of phosphorus reacts with...Ch. 5 - The empirical formula and molar mass of several...Ch. 5 - The malar mass and empirical formula of several...Ch. 5 - Combustion analysis of a hydrocarbon produced...Ch. 5 - Combustion analysis of naphthalene, a hydrocarbon...Ch. 5 - The foul odor of rancid butter is due largely to...Ch. 5 - Tartaric acid is the white, powdery substance that...Ch. 5 - Prob. 111ECh. 5 - Prob. 112ECh. 5 - Prob. 113ECh. 5 - Prob. 114ECh. 5 - How many molecules of ethanol (C2H5OH) (the...Ch. 5 - A drop of water has a volume of approximately 0.05...Ch. 5 - Determine the chemical formula of each compound...Ch. 5 - Determine the chemical formula of each compound...Ch. 5 - A Freon™ leak in the air conditioning system of an...Ch. 5 - Prob. 120ECh. 5 - A metal (M) forms a compound with the formula...Ch. 5 - A metal (M) forms an oxide with the formula M2O....Ch. 5 - Estradiol is a female sexual hormone that causes...Ch. 5 - Fructose is a common sugar found in fruit....Ch. 5 - Combustion analysis of a 13.42-g sample of equilin...Ch. 5 - Prob. 126ECh. 5 - Epsom salts is a hydrated ionic compound with the...Ch. 5 - A hydrate of copper(ll) chloride has the following...Ch. 5 - A compound of molar mass 177 g/mol contains only...Ch. 5 - Researchers obtain the following data from...Ch. 5 - Find the total number of atoms in a sample of...Ch. 5 - Vanadium forms four different oxides in which the...Ch. 5 - The chloride of an unknown metal is believed to...Ch. 5 - Prob. 134ECh. 5 - A chromium-containing compound has the formula...Ch. 5 - Prob. 136ECh. 5 - Prob. 137ECh. 5 - Prob. 138ECh. 5 - A mixture of NaCI and NaBr has a mass of 2.00 g...Ch. 5 - Three pure compounds form when 1.00-g samples of...Ch. 5 - A mixture of CaCO3 and (NH4)2CO3is 61.9% CO3 by...Ch. 5 - A mixture of 50.0 g of S and 1.00 x 102 g of CI2...Ch. 5 - Because of increasing evidence of damage to the...Ch. 5 - A particular coal contains 2.55% sulfur by mass....Ch. 5 - Lead is found in Earth’s crust as several...Ch. 5 - A 2.52-g sample of a compound containing only...Ch. 5 - Prob. 147ECh. 5 - The elements X and Y form a compound that is 40% X...Ch. 5 - A compound of X and Y is 13 X by mass. The atomic...Ch. 5 - A mixture of carbon and sulfur has a mass of 9.0...Ch. 5 - When molecules are represented by molecular...Ch. 5 - Prob. 152ECh. 5 - Explain the problem with this statement and...Ch. 5 - Without doing any calculations, arrange the...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 5. Write the formation reaction of the following complex compounds from the following reactants: 6. AgNO₃ + K₂CrO₂ + NH₄OH → 7. HgNO₃ + excess KI → 8. Al(NO₃)₃ + excess NaOH →arrow_forwardIndicate whether the product formed in the reaction exhibits tautomerism. If so, draw the structure of the tautomers. CO₂C2H5 + CH3-NH-NH,arrow_forwardDraw the major product of this reaction N-(cyclohex-1-en-1-yl)-1-(pyrrolidino) reacts with CH2=CHCHO, heat, H3O+arrow_forward
- Draw the starting material that would be needed to make this product through an intramolecular Dieckmann reactionarrow_forwardDraw the major product of this reaction. Nitropropane reacts + pent-3-en-2-one reacts with NaOCH2CH3, CH3CHOHarrow_forwardIndicate whether the product formed in the reaction exhibits tautomerism. If so, draw the structure of the tautomers. OC2H5 + CoHs-NH-NH,arrow_forward
- Explain how substitutions at the 5-position of barbituric acid increase the compound's lipophilicity.arrow_forwardExplain how substitutions at the 5-position of phenobarbital increase the compound's lipophilicity.arrow_forwardName an interesting derivative of barbituric acid, describing its structure.arrow_forward
- Briefly describe the synthesis mechanism of barbituric acid from the condensation of urea with a β-diketone.arrow_forwardGiven the hydrazones indicated, draw the structures of the enamines that can be formed. Indicate the most stable enamine (explain). C6H5 C6H5 H C6H5 Harrow_forward4. Propose a Synthesis for the molecule below. You may use any starting materials containing 6 carbons or less (reagents that aren't incorporated into the final molecule such as PhзP do not count towards this total, and the starting material can have whatever non-carbon functional groups you want), and any of the reactions you have learned so far in organic chemistry I, II, and III. Your final answer should show each step separately, with intermediates and conditions clearly drawn.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY