
A ?owchart of a methanol synthesis process is shown below.
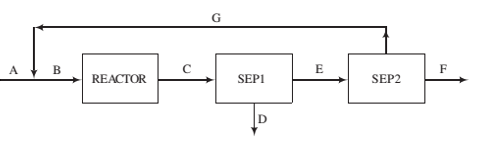
The following speci?cations apply to the labeled streams and process units:
A. Fresh feed—a mixture of CO, H2, N2, and CO2
B. Feed to the reactor—30.0 mole% CO, 63.0% H2, 2.0% N2, and 5.0% CO2.
Reactor. Two reactions occur and proceed to equilibrium at 200°C and 4925 kPa absolute:
C. Reactor effluent—contains all feed and product species at the reactor temperature and pressure. Species partial pressures satisfy the two given equations.
Sep1. Condenses all methanol and water in reactor ef?uent.
D. Liquid methanol and water, (These species will be separated by distillation in a unit not shown.)
E. Gas containing N2and unreacted CO, H2, and CO2.
Sep2. Multiple-unit separation process.
F. All of the nitrogen and some of the hydrogen in Stream E.
G. Recycle stream—CO, CO2, and 10% of the hydrogen fed to Sep2.
(a) Taking 100 kmol/h of Stream B as a basis of calculation, calculate the molar ?ow rates (kmol/h) and molar compositions of the remaining six labeled streams.
(b) The process is to be used to provide 237 kmol/h of methanol. Scale up the ?owchart of Part (a) to calculate the required fresh feed rate (SCMH), the ?ow rate of the reactor ef?uent (SCMH), and the actual volumetric ?ow rate of the reactor ef?uent (m3/h), assuming ideal-gas behavior.
(c) Use the rule of thumb for a diatomic gas given in Equation 5.2-3 to test the ideal-gas assumption at the reactor outlet. If the assumption is invalid, which of the values calculated in Part (b) are in error?
Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
ELEMENTARY PRINCIPLES OF CHEM. PROCESS.
Additional Science Textbook Solutions
Elementary Surveying: An Introduction To Geomatics (15th Edition)
INTERNATIONAL EDITION---Engineering Mechanics: Statics, 14th edition (SI unit)
Starting Out with C++ from Control Structures to Objects (9th Edition)
Starting Out with Python (4th Edition)
Introduction To Programming Using Visual Basic (11th Edition)
Problem Solving with C++ (10th Edition)
- Emergency response for the rupture of an ammonia pipeline is being planned. Themaximum estimated flow rate from the rupture is 20 kg/s. Local authorities havedetermined that evacuations are necessary if the concentration exceeds the ERPG-2 level.Assume a temperature of 20˚C, wind speed of 3 m/s, atmospheric pressure of 1 atm, 70%cloud cover and rural conditions. State any other assumptions.a. How far directly downwind needs to be evacuated?b. Using a spreadsheet (such as excel), draw a plot of the isopleth at thisconcentration. You should have at least 8 different distances downwind markedon your plot.arrow_forwardA reactor in a pesticide plant contains 8000 lbm of a liquid mixture of 50% by weightmethyl isocyanate (MIC). The liquid is near its boiling point. A study of various releasescenarios indicates that a rupture of the reactor will spill the liquid to a boiling pool onthe ground. The boiling rate of the MIC has been estimated to be 50 lb m/min. Evacuationof the population must occur in areas where the vapor concentration exceeds ERPG-3levels. If the wind speed is 10 mph on a clear winter night, estimate the distancedownwind that must be evacuated.arrow_forwardA burning dump emits an estimated 1.5 kg/min of nitrogen dioxide (NO2 ). On a partlycloudy morning with a 2.5 m/s wind and temperature of 18°C, what is the concentrationof NO2 at a distance of 3.0 km directly downwind of the dump? Does this exceed theshort-term exposure limit for NO2 ? State your assumptions.arrow_forward
- For each set of measurements below, calculate the Grubbs statistic, G, look up the appropriate critical value of G from Table 4.6, and determine whether the Grubbs test supports discarding the first value in the list at the 95% level of confidence. a) 106.0, 165.0, 167.5, 170.5, 163.5, 170.7 (Geale -2.028; Gerit 1.822; yes, the Grubbs test supports discarding 106.0) b) 214.8, 263.0, 229.9, 236.9, 221.8, 230.8, 241.1 c) 357.0, 309.3, 304.9, 314.8, 305.8, 295.3, 284.7, 299.5 TABLE 4-6 Critical values of G for rejection of outlier Number of observations otsulsve os Tenos nagsibarito G to buboxy (95% confidence) 456 1.463 1.672 1.822 7 1.938 8 upa 2.032 9 2.110 10 2.176 - 1 12 15 20 11 2.234 2.285 2.409 2.557arrow_forward#1 A irreversible isothermal gas-phase isomerization reaction is given as: AB. This reaction is conducted in a 400L batch reactor and 100 mol of A (NAD = 100 mol) is charged into this reactor. The rate of reaction is determined as a function of the conversion of reactant A and the results are given below. The temperature was constant at 500K and the total pressure was constant at 830 kPa. The entering number of moles of species A is 100 mol. Calculate the time necessary to achieve 80% conversion. 0 0.1 0.2 0.4 -TA (mol/m³.s) 0.45 0.37 0.3 0.195 0.6 0.113 0.7 0.079 0.8 0.05arrow_forward#3 A irreversible isothermal liquid-phase reaction is given as: A → B is conducted in continuous flow systems. The rate of reaction is determined as a function of the conversion of reactant A and the results are given below. The temperature was constant at 500K. The entering molar flow rate of A is 0.4 mol/min. a) If this reaction is conducted in two CSTRS in series. Calculate the required reactor volume of each CSTRS if conversion X₁ = 0.4 and conversion X2 = 0.8. b) If this reaction is conducted in two PFRS in series. Calculate the required reactor volume of each PFRS if conversion X₁ = 0.4 and conversion X2 = 0.8. c) If this reaction is conducted in a PFR followed by a CSTR. Calculate the required reactor volume of PFR if conversion X₁ = 0.4 and of CSTR if conversion X2 = 0.8. X -A (mol/L.min) 0 0.1 0.2 0.4 0.6 0.7 0.8 0.45 0.37 0.3 0.195 0.113 0.079 0.05arrow_forward
- #2 An exothermic reaction, AB + C, was carried out adiabatically in a PFR or a CSTR and the following data was recorded. The entering molar flow rate of A was 300 mol/min. Calculate the necessary i) PFR volume and ii) CSTR volume to achieve 40% conversion. X 0 0.2 0.4 0.45 0.5 0.6 0.8 0.9 -TA (mol/L-min) 1 1.67 5 5 5 5 1.25 0.91arrow_forwardQuestion: McDaniel Shipyards wants to develop control charts to assess the quality of its steel plate. They... McDaniel Shipyards wants to develop control charts to assess the quality of its steel plate. They take ten sheets of 1" steel plate and compute the number of cosmetic flaws on each roll. Each sheet is 20' by 100'. Compute within 99.73% control limits. Based on the following data: a. Develop limits for the control chart b. Is the process in or out of control? c. Can you detect any outliers, if so which value(s)? Number of Sheet flaws 1 1 2 1 3 2 4 0 5 1 6 5 7 0 8 2 9 0 10 2arrow_forwardQuestion: McDaniel Shipyards wants to develop control charts to assess the quality of its steel plate. They take ten sheets of 1" steel plate and compute the number of cosmetic flaws on eac... McDaniel Shipyards wants to develop control charts to assess the quality of its steel plate. They take ten sheets of 1" steel plate and compute the number of cosmetic flaws on each roll. Each sheet is 20' by 100'. Based on the following data, develop limits for the control chart, plot the control chart, and determine whether the process is in control. Answer the following questions below. Number of flaws Sheet 1 1 2 1 = 3 2 4 0 5 1 6 5 7 0 8 2 9 10 0 2 PLEASE WRTIE NEATLY AND EXPLAIN! (: Thanks 1. Calculate the standard deviation of control chart. (a) the standard deviation = 1.0832 (b) the standard deviation = 1.1832 (c) the standard deviation = 1.4 (d) the standard deviation = 1.04 27. 2. Using +- 3 olimits, calculate the LCL and UCL for these data. 3.549; LCL = -3.549 (a) UCL (b) UCL 3.549;…arrow_forward
- Derive an expression for incompressible flow in a horizontal pipe of constant diameter andwithout fittings or valves which shows that the pressure is a linear function of pipe length. Whatother assumptions are required for this result? Is this result valid for non-horizontal pipes? Howwill the presence of fittings, valves and other hardware affect this result?arrow_forwardEthylene glycol liquid is used as an antifreeze in many applications. If it is stored in a vessel at a pressure of at 150 psig flows through a ¾ inch-diameter hole to atmospheric pressure. Estimate the discharge rate if the ambient pressure is 1 atm. For ethylene glycol at 77°F, the specific gravity is 1.15 and the viscosity is 25 cP. The molecular weight is 62.07.arrow_forwardPlease help me with parts A through Darrow_forward
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





