
PKG ORGANIC CHEMISTRY
5th Edition
ISBN: 9781259963667
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 5, Problem 5.5P
A molecule is achiral if it has a plane of symmetry in any conformation. Each of the following conformations does not have a plane of symmetry, but rotation around a carbon-carbon bond forms a conformation that does have a plane of symmetry. Draw this conformation for each molecule.
a. 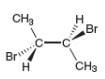 b.
b. 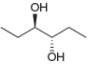
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
>
each pair of substrates below, choose the one that will react faster in a substitution reaction, assuming that:
1. the rate of substitution doesn't depend on nucleophile concentration and
2. the products are a roughly 50/50 mixture of enantiomers.
Substrate A
Substrate B
Faster Rate
X
Ś
CI
(Choose one)
(Choose one)
CI
Br
Explanation
Check
Br
(Choose one)
© 2025 McGraw Hill LLC. All Rights F
NMR spectrum of ethyl acetate has signals whose chemical shifts are indicated below. Which hydrogen or set of hydrogens corresponds to the signal at
4.1 ppm? Select the single best answer.
The
H
O
HỌC—C—0—CH, CH,
2
A
ethyl acetate
H NMR: 1.3 ppm, 2.0 ppm, 4.1 ppm
Check
OA
B
OC
ch
B
C
Save For Later
Submit Ass
© 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center |
How many signals do you expect in the H NMR spectrum for this molecule?
Br Br
Write the answer below.
Also, in each of the drawing areas below is a copy of the molecule, with Hs shown. In each copy, one of the H atoms is colored red. Highlight in red all other H
atoms that would contribute to the same signal as the H already highlighted red
Note for advanced students: In this question, any multiplet is counted as one signal.
1
Number of signals in the 'H NMR spectrum.
For the molecule in the top drawing area, highlight in red any other H atoms that will contribute to
the same signal as the H atom already highlighted red.
If no other H atoms will contribute, check the box at right.
Check
For the molecule in the bottom drawing area, highlight in red any other H atoms that will contribute
to the same signal as the H atom already highlighted red.
If no other H atoms will contribute, check the box at right.
O
✓
No additional Hs to color in top
molecule
ง
No additional Hs to color in bottom…
Chapter 5 Solutions
PKG ORGANIC CHEMISTRY
Ch. 5 - Prob. 5.1PCh. 5 - Prob. 5.2PCh. 5 - Draw the mirror image of each compound. Label each...Ch. 5 - Prob. 5.4PCh. 5 - A molecule is achiral if it has a plane of...Ch. 5 - Problem 5.6 Locate the stereogenic centers in each...Ch. 5 - The facts in section 5.4A can be used to locate...Ch. 5 - Prob. 5.8PCh. 5 - Prob. 5.9PCh. 5 - Prob. 5.10P
Ch. 5 - Prob. 5.11PCh. 5 - Prob. 5.12PCh. 5 - Label each compound as R or S.Ch. 5 - Prob. 5.14PCh. 5 - Prob. 5.15PCh. 5 - Prob. 5.16PCh. 5 - Prob. 5.17PCh. 5 - Problem 5.18 Compounds E and F are two isomers of...Ch. 5 - Prob. 5.19PCh. 5 - Prob. 5.20PCh. 5 - Prob. 5.21PCh. 5 - Prob. 5.22PCh. 5 - Prob. 5.23PCh. 5 - Prob. 5.24PCh. 5 - Which of the following cyclic molecules are meso...Ch. 5 - Prob. 5.26PCh. 5 - Prob. 5.27PCh. 5 - Problem 5.28 The amino acid has the physical...Ch. 5 - Prob. 5.29PCh. 5 - Prob. 5.30PCh. 5 - Prob. 5.31PCh. 5 - Prob. 5.32PCh. 5 - Prob. 5.33PCh. 5 - Prob. 5.34PCh. 5 - Prob. 5.35PCh. 5 - Prob. 5.36PCh. 5 - Prob. 5.37PCh. 5 - Prob. 5.38PCh. 5 - Prob. 5.39PCh. 5 - 5.40 Determine if each compound is identical to or...Ch. 5 - Prob. 5.41PCh. 5 - Prob. 5.42PCh. 5 - Prob. 5.43PCh. 5 - Prob. 5.44PCh. 5 - Prob. 5.45PCh. 5 - Prob. 5.46PCh. 5 - Label each stereogenic center as R or S. a. c. e....Ch. 5 - Prob. 5.48PCh. 5 - Prob. 5.49PCh. 5 - Prob. 5.50PCh. 5 - Prob. 5.51PCh. 5 - Prob. 5.52PCh. 5 - Prob. 5.53PCh. 5 - Prob. 5.54PCh. 5 - Prob. 5.55PCh. 5 - Prob. 5.56PCh. 5 - Prob. 5.57PCh. 5 - Prob. 5.58PCh. 5 - 5.59 Explain each statement by referring to...Ch. 5 - Prob. 5.60PCh. 5 - Prob. 5.61PCh. 5 - Prob. 5.62PCh. 5 - Prob. 5.63PCh. 5 - Prob. 5.64PCh. 5 - Prob. 5.65PCh. 5 - Prob. 5.66PCh. 5 -
5.67 Artemisinin and mefloquine are widely used...Ch. 5 - 5.68 Saquinavir (trade name Invirase) is a...Ch. 5 - Prob. 5.69PCh. 5 - Prob. 5.70PCh. 5 - Prob. 5.71PCh. 5 - Prob. 5.72PCh. 5 - Problem 5.73 An acid-base reaction of with a...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Some people consider Pasteur or Koch to be the Father of Microbiology, rather than Leeuwenhoek. Why might they ...
Microbiology with Diseases by Body System (5th Edition)
11. In the early 1800s, French naturalist Jean Baptiste Lamarck suggested that the best explanation for the rel...
Campbell Biology: Concepts & Connections (9th Edition)
What process causes the Mediterranean intermediate Water MIW to become more dense than water in the adjacent At...
Applications and Investigations in Earth Science (9th Edition)
Sea turtles have disappeared from many regions, and one way of trying to save them is to reintroduce them to ar...
MARINE BIOLOGY
Give the IUPAC name for each compound.
Organic Chemistry
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- in the kinetics experiment, what were the values calculated? Select all that apply.a) equilibrium constantb) pHc) order of reactiond) rate contstantarrow_forwardtrue or false, given that a 20.00 mL sample of NaOH took 24.15 mL of 0.141 M HCI to reach the endpoint in a titration, the concentration of the NaOH is 1.17 M.arrow_forwardin the bromothymol blue experiment, pKa was measured. A closely related compound has a Ka of 2.10 x 10-5. What is the pKa?a) 7.1b) 4.7c) 2.0arrow_forward
- calculate the equilibrium concentration of H2 given that K= 0.017 at a constant temperature for this reaction. The inital concentration of HBr is 0.050 M.2HBr(g) ↔ H2(g) + Br2(g)a) 4.48 x 10-2 M b) 5.17 x 10-3 Mc) 1.03 x 10-2 Md) 1.70 x 10-2 Marrow_forwardtrue or falsegiven these two equilibria with their equilibrium constants:H2(g) + CI2(l) ↔ 2HCI(g) K= 0.006 CI2(l) ↔ CI2(g) K= 0.30The equilibrium contstant for the following reaction is 1.8H2(g) + CI2 ↔ 2HCI(g)arrow_forwardI2(g) + CI2(g) ↔ 2ICIK for this reaction is 81.9. Find the equilibrium concentration of I2 if the inital concentration of I2 and CI2 are 0.010 Marrow_forward
- true or false,the equilibrium constant for this reaction is 0.50.PCI5(g) ↔ PCI3(g) + CI2(g)Based on the above, the equilibrium constant for the following reaction is 0.25.2PCI5(g) ↔. 2PCI3(g) + 2CI2(g)arrow_forwardtrue or false, using the following equilibrium, if carbon dioxide is added the equilibrium will shift toward the productsC(s) + CO2(g) ↔ 2CO(g)arrow_forward2S2O2/3- (aq) + I2 (aq) ---> S4O2/6- (aq) +2I- (aq) Experiment I2 (M) S2O3- (M) Initital Rate (M/s) 1 0.01 0.01 0.0004 2 0.01 0.02 0.0004 3 0.02 0.01 0.0008 Calculate the overall order for this reaction using the table data a) 3b) 0c) 2d) 1arrow_forward
- the decomposition of N2O5 is the first order with a half-life of 1.98 minutes. If the inital concentration of N2O5 is 0.200 M, what is the concentration after 6 minutes?a) 0.612 Mb) 0.035 Mc) 0.024 Md) 0.100 Marrow_forward20.00 mL of 0.150 M HCI is titrated with 0.075 M NaOH. What volume of NaOH is needed?a) 50 mLb) 20 mLc) 40 mLd) 26.66 mLarrow_forward20.00 mL of 0.150 M NaOH is titrated with 37.75 mL of HCI. What is the molarity of the HCI?a) 0.150 Mb) 0.079 Mc) 0.025 Md) 0.050 Marrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License