
Concept explainers
(a)
Interpretation:
A verification whether the molar conductivity follows the Kohlrausch law has to be done. The value of the limiting molar conductivity has to be calculated.
Concept Introduction:
Kohlrausch law:
The molar conductivity of an electrolyte at infinite dilution is equal to the sum of the individual conductances of the anions and cations.
(a)
Answer to Problem 5.56P
The limiting molar conductivity is found to be
Explanation of Solution
According to Kohlrausch law, Molar conductivity of a strong electrolyte weakly depends on concentration. On dilution, there is a regular increase in the molar conductivity, due to the decrease in solute-solute interaction.
Molar conductivity can be mathematically represented as
Where,
Substituting the first set of values and cell constant in the above equation and solving for
Similarly, the rest of the calculation can be done as shown above.
It is clear from the table that molar conductivity of a strong electrolyte weakly depends on concentration as all the values came nearly same. Also it shows that on dilution, there is a regular increase in the molar conductivity.
The graph between
Where,
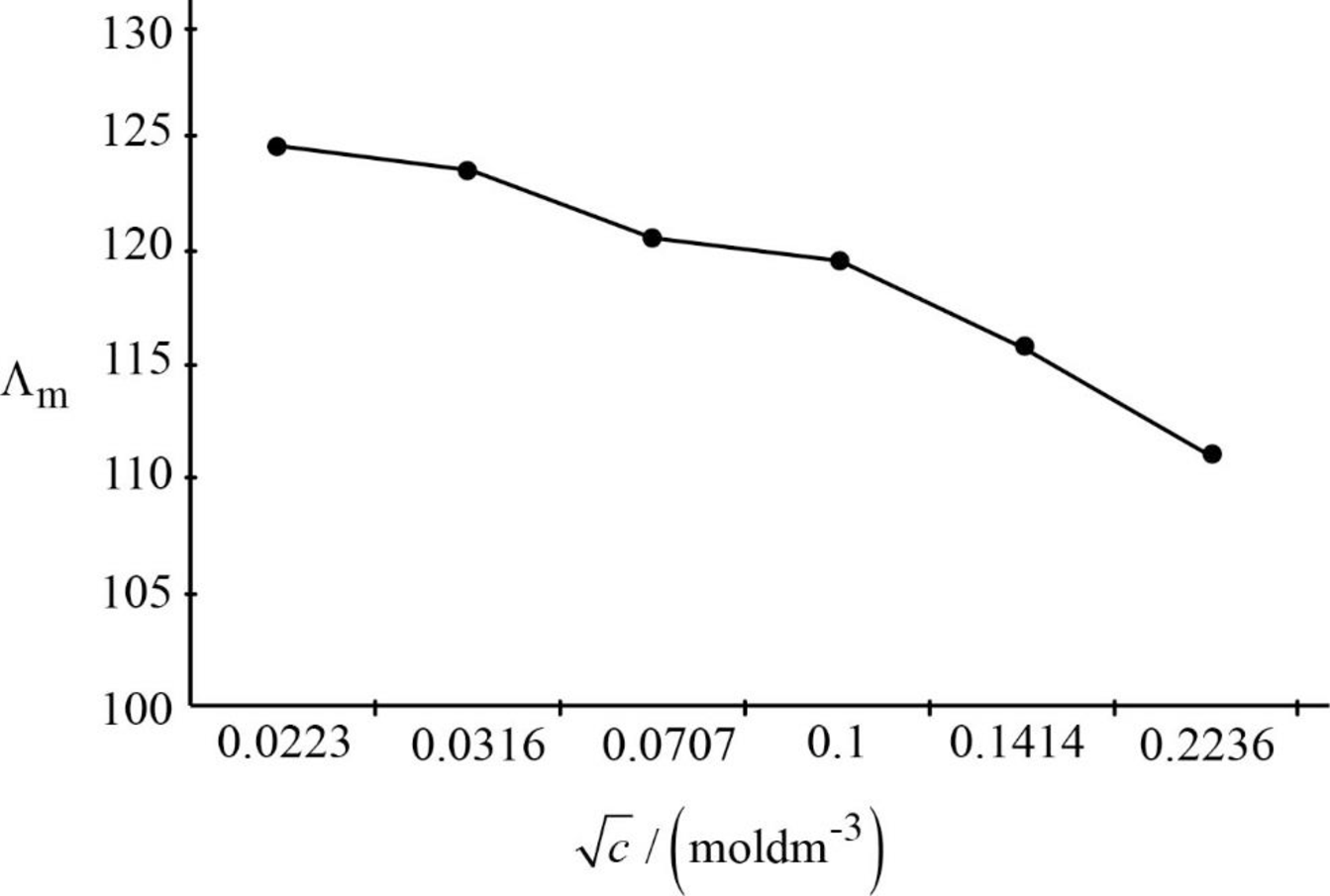
Figure.1
Upon extrapolation the graph, where it will touch on the Y-axis that will be the limiting molar conductivity. Hence, the limiting molar conductivity is around
(b)
Interpretation:
The value of coefficient
Concept Introduction:
Kohlrausch law:
The molar conductivity of an electrolyte at infinite dilution is equal to the sum of the individual conductances of the anions and cations.
(b)
Answer to Problem 5.56P
The value of coefficient
Explanation of Solution
The slope of the graph between
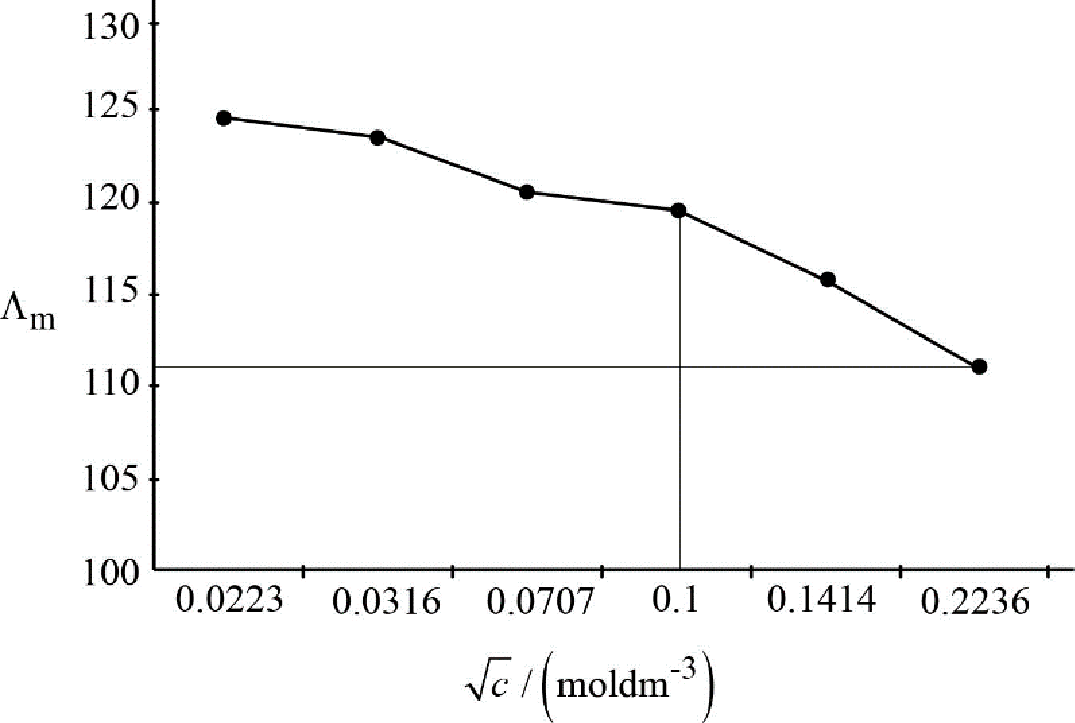
Figure.2
Slope of the graph:
After considering carefully, the unit of
Therefore, the value of coefficient
(c)
Interpretation:
The molar conductivity, conductivity and the resistance of
Concept Introduction:
Kohlrausch law:
The molar conductivity of an electrolyte at infinite dilution is equal to the sum of the individual conductances of the anions and cations.
(c)
Answer to Problem 5.56P
The molar conductivity, conductivity and the resistance of
Explanation of Solution
(I)
Given Data:
The limiting molar conductivity of
Now, molar conductivity
Therefore, the molar conductivity of
(II)
Calculation of conductivity:
Molar conductivity can be mathematically represented as
Where,
Hence, the conductivity can be calculated as
Therefore, the conductivity of
(III)
The conductivity can be further simplified as
Hence, the resistance can be calculated as
Therefore, the resistance of
Want to see more full solutions like this?
Chapter 5 Solutions
Elements Of Physical Chemistry
- Ideally nonpolarizable electrodes can1. participate as reducers in reactions.2. be formed only with hydrogen.3. participate as oxidizers in reactions.4. form open and closed electrochemical systems.arrow_forwardIndicate the options for an electrified interface:1. Temperature has no influence on it.2. Not all theories that describe it include a well-defined electrical double layer.3. Under favorable conditions, its differential capacitance can be determined with the help of experimental measurements.4. A component with high electronic conductivity is involved in its formation.arrow_forwardTo describe the structure of the interface, there are theories or models that can be distinguished by:1. calculation of the charge density.2. distribution of ions in the solution.3. experimentally measured potential difference.4. external Helmoltz plane.arrow_forward
- Indicate the correct options when referring to Luther's equation:1. It is not always easy to compare its results with experimental results.2. It depends on the number of electrons exchanged in the species involved.3. Its foundation is thermodynamic.4. The values calculated with it do not depend on temperature.arrow_forwardIndicate which of the unit options correspond to a measurement of current density.1. A s m-22. mC s-1 m-23. Ω m-24. V J-1 m-2arrow_forwardIndicate the options that are true when referring to electrode membranes:1. The Donnan potential, in general, does not always intervene in membranes.2. There are several ways to classify the same membrane.3. Any membrane can be used to determine the pH of a solution.4. Only one solution and one membrane are needed to determine the pH of that solution.arrow_forward
- Calculate the maximum volume of carbon dioxide gasarrow_forwardIn galvanic cells, their potential1. can be measured with a potentiometer2. does not depend on the equilibrium constant of the reaction occurring within them3. is only calculated from the normal potentials of the electrodes they comprise4. can sometimes be considered a variation in a potential differencearrow_forwardIf some molecules in an excited state collide with other molecules in a ground state, this process1. can occur in solution and in the gas phase.2. can be treated as a bimolecular process.3. always results in collisional deactivation.4. does not compete with any other process.arrow_forward
- Radiation of frequency v is incident on molecules in their ground state. The expected outcome is that1. the molecules do not change their state.2. the molecules transition to an excited state.3. the molecules undergo a secondary process.4. collisional deactivation occurs.arrow_forwardPredict the major product of the following reaction and then draw a curved arrow mechanism for its formation. Part: 0/2 Part 1 of 2 H₂SO heat : OH 90 Draw the structure of the major product. Click and drag to start drawing a structure. 3arrow_forwardDraw a curved arrow mechanism for the reaction, adding steps as necessary. Be sure to include all electrons that are necessary to the mechanism and all nonzero formal charges. C Ö-H H + -S-OH .0. Add/Remove step X टे Click and drag to start drawing a structure.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





