
Concept explainers
(a)
Interpretation: The structure of naturally occurring
Concept introduction: A compound exhibits number of stereoisomers when it contains more than one stereogenic centers. The maximum number of stereoisomers a compound with
Answer to Problem 5.51P
The structure of naturally occurring
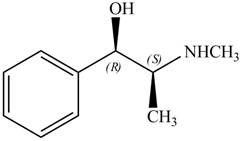
Explanation of Solution
The structure of given compoundis shown below.
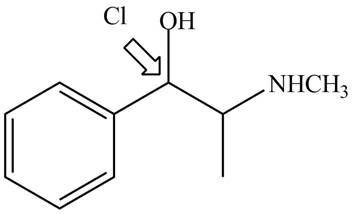
Figure 1
The stereochemistry of the compound is determined by prioritizing the groups attached to its stereogenic center. The groups are prioritizedon the basis ofatomic number of their atoms. The group that contains atom with a the higher
The configuration at the first stereocenter of
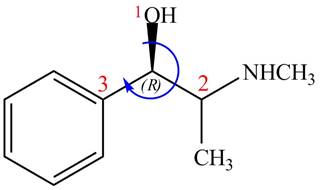
Figure 2
The above structure implies that the configuration at the first stereocenter of
The configuration at the second stereocenter of
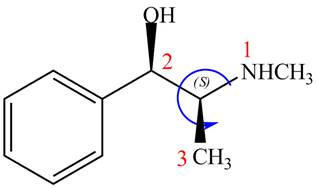
Figure 3
The above structure implies that the configuration at the second stereocenter of
The configuration at both the stereocenters of
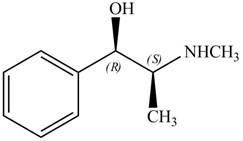
Figure 4
The configurations at the first and second stereocenters of
The structure of naturally occurring
(b)
Interpretation: The structure of naturally occurring
Concept introduction: A compound exhibits number of stereoisomers when it contains more than one stereogenic centers. The maximum number of stereoisomers a compound with
Answer to Problem 5.51P
The structure of naturally occurring
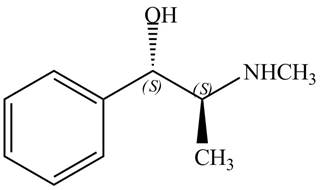
Explanation of Solution
The structure of naturally occurring
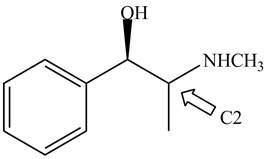
Figure 5
The stereochemistry of the compound is determined by prioritizing the groups attached to its stereogenic center. The groups are prioritizedon the basis of atomic number of their atoms. The group that contain atom with higher atomic number is givenhigher priority. Complete the circle in decreasing order of priorityfrom
The configuration at first chiral center of
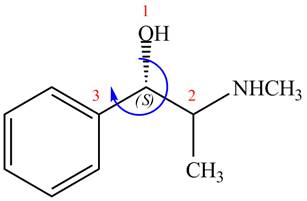
Figure 6
The above structure implies that the configuration at the first stereocenter of
The configuration at the second chiral center of
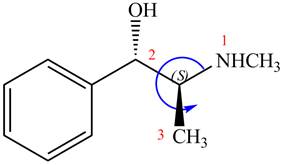
Figure 7
The above structure implies that the configuration at the second stereocenter of
The configuration at the first and second chiral centers of
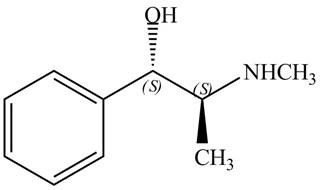
Figure 8
The configurations at the first and second carbon atoms of
The structure of naturally occurring
(c)
Interpretation: The relationship between ephedrine and pseudoephedrine is to be identified.
Concept introduction: A compound exhibits number of stereoisomers when it contains more than one stereogenic centers. The maximum number of stereoisomers a compound with
Answer to Problem 5.51P
Ephedrine and pseudoephedrine are related as diastereomers.
Explanation of Solution
The relationship between ephedrine and pseudoephedrine is shown below.
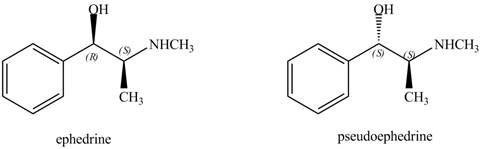
Figure 9
One stereocenter in both the compounds has opposite configuration, while the other stereogenic center has the same configuration. Therefore, both the compounds are related as diastereomer.
Ephedrine and pseudoephedrine are related as diastereomers.
(d)
Interpretation: All the stereoisomers of
Concept introduction: A compound exhibits number of stereoisomers when it contains more than one stereogenic centers. The maximum number of stereoisomers a compound with
Answer to Problem 5.51P
The stereoisomers of
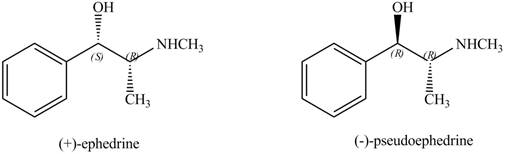
Explanation of Solution
The stereoisomers of
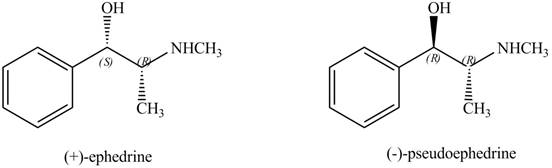
Figure 10
Both the stereoisomers have two stereocenters. One stereocenter in both the compounds has the same configuration, while the other stereocenter in both the compounds has opposite configuration.
The stereoisomers of
(e)
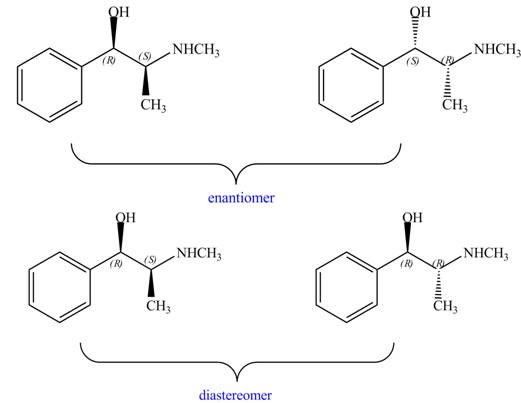
Interpretation: The relationship between each compound drawn in part (d) and
Concept introduction: A compound exhibits number of stereoisomers when it contains more than one stereogenic centers. The maximum number of stereoisomers a compound with
Answer to Problem 5.51P
The relationship between each compound drawn in part (d) and
Explanation of Solution
The relationship between each compound drawn in part (d) and

Figure 11
The compounds
The relationship between each compound drawn in part (d) and
Want to see more full solutions like this?
Chapter 5 Solutions
Organic Chemistry-Package(Custom)
- What is the product of the reaction? F3C. CF3 OMe NaOH / H₂Oarrow_forwardWhat would you expect to be the major product obtained from the following reaction? Please explain what is happening here. Provide a detailed explanation and a drawing showing how the reaction occurs. The correct answer to this question is V.arrow_forwardPlease answer the question for the reactions, thank youarrow_forward
- What is the product of the following reaction? Please include a detailed explanation of what is happening in this question. Include a drawing showing how the reagent is reacting with the catalyst to produce the correct product. The correct answer is IV.arrow_forwardPlease complete the reactions, thank youarrow_forwardConsider the synthesis. What is compound Y? Please explain what is happening in this question. Provide a detailed explanation and a drawing to show how the compound Y creates the product. The correct answer is D.arrow_forward
- What would be the major product of the following reaction? Please include a detailed explanation of what is happening in this question. Include steps and a drawing to show this reaction proceeds and how the final product is formed. The correct answer is B. I put answer D and I don't really understand what is going on in the question.arrow_forwardWhat is the product of the following reaction? Please explain what is happening in this question. Provide a detailed explanation and a drawing showing how the reagent is reacting with the catalysts to product the correct product. The correct answer is B.arrow_forwardWhat is the missing intermediate 1 and the final product 2. Please include a detailed explanation explaining the steps of malonic ester synthesis. Please include drawings of the intermediate and how it occurs and how the final product is former.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

