
(a)
Interpretation:
The preparation of the given compound from the alkene and other reagents is to be stated.
Concept introduction:
The systematic naming of organic compound is given by
Answer to Problem 5.32AP
The alkene which is used to synthesize the given compound is shown below.
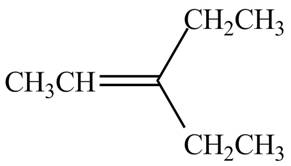
The synthesis of the given compound is shown below.
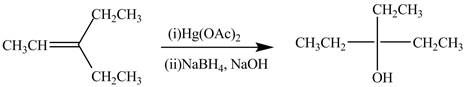
Explanation of Solution
The alkene which is used to synthesize the given compound is shown below.

Figure 1
The alkene
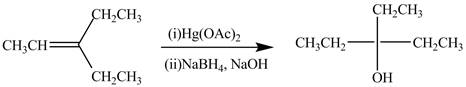
Figure 2
The preparation of the given compound from the alkene and other reagents is shown in Figure 2.
(b)
Interpretation:
The preparation of the given compound from the alkene with and other reagents is to be stated.
Concept introduction:
Alkenes are the unsaturated class of organic compounds which have a double bond in their structure. The general formula of alkene is written as
The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of the organic compound is correctly interpreted from the name
Answer to Problem 5.32AP
The alkene which is used to synthesize the given compound is shown below.
![]()
The synthesis of the given compound is shown below.
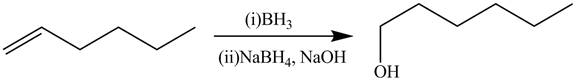
Explanation of Solution
The alkene which is used to synthesize the given compound is shown below.
![]()
Figure 3
The alkene
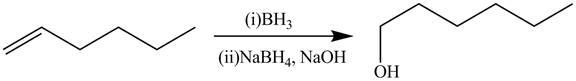
Figure 4
The preparation of the given compound from the alkene and other reagents is shown in Figure 4.
(c)
Interpretation:
The preparation of the given compound from the alkene and other reagents is to be stated.
Concept introduction:
Alkenes are the unsaturated class of organic compounds which have a double bond in their structure. The general formula of alkene is written as
The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of the organic compound is correctly interpreted from the name.
Answer to Problem 5.32AP
The alkene which is used to synthesize the given compound is shown below.
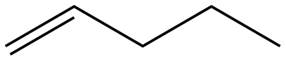
The synthesis of the given compound is shown below.
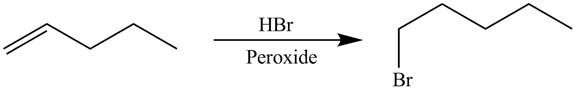
Explanation of Solution
The alkene which is used to synthesize the given compound is shown below.

Figure 5
The alkene

Figure 6
The preparation of the given compound from the alkene and other reagents is shown in Figure 6.
(d)
Interpretation:
The preparation of the given compound from the alkene and other reagents is to be stated.
Concept introduction:
Alkenes are the unsaturated class of organic compounds which have a double bond in their structure. The general formula of alkene is written as
The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of the organic compound is correctly interpreted from the name.
Answer to Problem 5.32AP
The alkene which is used to synthesize the given compound is shown below.
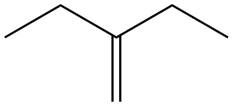
The synthesis of the given compound is shown below.
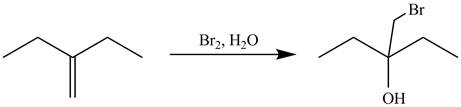
Explanation of Solution
The alkene which is used to synthesize the given compound is shown below.
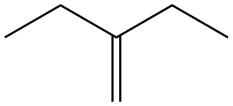
Figure 7
The alkene
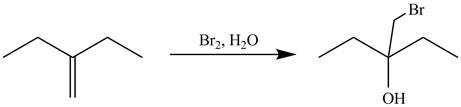
Figure 8
The preparation of the given compound from the alkene and other reagents is shown in Figure 8.
(e)
Interpretation:
The preparation of the given compound from the alkene and other reagents is to be stated.
Concept introduction:
Alkenes are the unsaturated class of organic compounds which have a double bond in their structure. The general formula of alkene is written as
The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of the organic compound is correctly interpreted from the name.
Answer to Problem 5.32AP
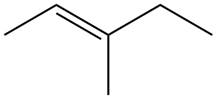
The alkene which is used to synthesize the given compound is shown below.
The synthesis of the given compound is shown below.
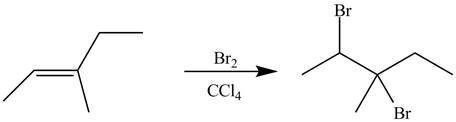
Explanation of Solution
The alkene which is used to synthesize the given compound is shown below.

Figure 9
The alkene
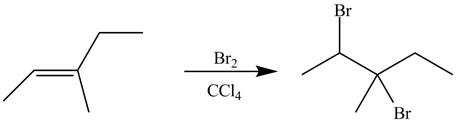
Figure 10
The preparation of the given compound from the alkene and other reagents is shown in Figure 10.
(f)
Interpretation:
The preparation of the given compound from the alkene and other reagents is to be stated.
Concept introduction:
Alkenes are the unsaturated class of organic compounds which have a double bond in their structure. The general formula of alkene is written as
The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of the organic compound is correctly interpreted from the name.
Answer to Problem 5.32AP
The alkene which is used to synthesize the given compound is shown below.
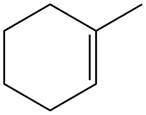
The synthesis of the given compound is shown below.
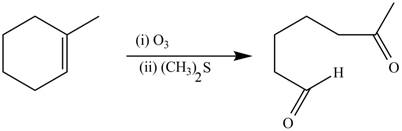
Explanation of Solution
The alkene which is used to synthesize the given compound is shown below.
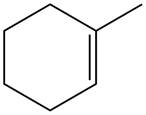
Figure 11
The alkene

Figure 12
The preparation of the given compound from the alkene and other reagents is shown in Figure 12.
(g)
Interpretation:
The preparation of the given compound from the alkene and other reagents is to be stated.
Concept introduction:
Alkenes are the unsaturated class of organic compounds which have a double bond in their structure. The general formula of alkene is written as
The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of the organic compound is correctly interpreted from the name.
Answer to Problem 5.32AP
The alkene which is used to synthesize the given compound is shown below.
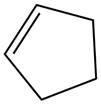
The synthesis of the given compound is shown below.
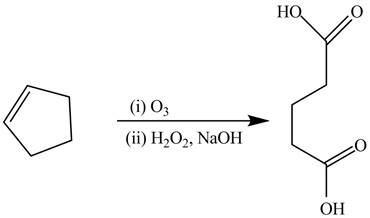
Explanation of Solution
The alkene which is used to synthesize the given compound is shown below.

Figure 13
The alkene cyclopentene undergoes ozonolysis reaction followed by oxidation reaction in presence of
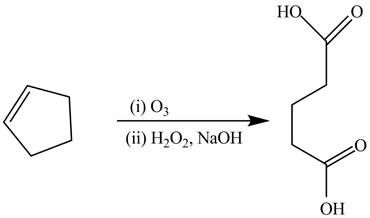
Figure 14
The preparation of the given compound from the alkene and other reagents is shown in Figure 14.
(h)
Interpretation:
The preparation of the given compound from the alkene and other reagents is to be stated.
Concept introduction:
Alkenes are the unsaturated class of organic compounds which have a double bond in their structure. The general formula of alkene is written as
The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of the organic compound is correctly interpreted from the name.
Answer to Problem 5.32AP
The alkene which is used to synthesize the given compound is shown below.
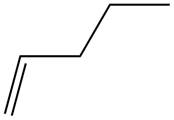
The synthesis of the given compound is shown below.
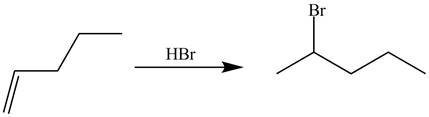
Explanation of Solution
The alkene which is used to synthesize the given compound is shown below.
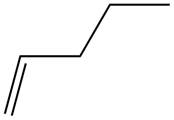
Figure 15
The alkene

Figure 16
The preparation of the given compound from the alkene and other reagentsis shown in Figure 16.
(i)
Interpretation:
The preparation of the given compound from the alkene and other reagents is to be stated.
Concept introduction:
Alkenes are the unsaturated class of organic compounds which have a double bond in their structure. The general formula of alkene is written as
The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of the organic compound is correctly interpreted from the name.
Answer to Problem 5.32AP
The alkene which is used to synthesize the given compound is shown below.
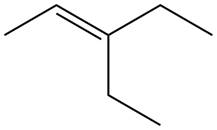
The synthesis of the given compound is shown below.
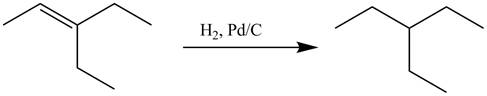
Explanation of Solution
The alkene which is used to synthesize the given compound is shown below.

Figure 17
The alkene
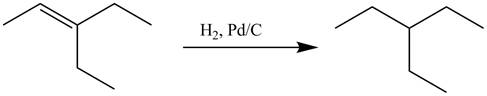
Figure 18
The preparation of the given compound from the alkene and other reagents is shown in Figure 18.
Want to see more full solutions like this?
Chapter 5 Solutions
ORGANIC CHEMISTRY SAPLING ACCESS + ETEX
- I have a aqueous solution (175 ml) of iridium trichloride containing 8,750 ppm Iridium by ICP OES analysis. What is the percent concentration of Iridium trichloride in aquous solution and provide the concentration in moles per liter, percentage by weight.arrow_forwardno Ai walkthroughsarrow_forwardno Ai walkthroughsarrow_forward
- no Ai walkthroughs (product in picture is wrong btw don't submit the same thing)arrow_forwardno Ai walkthroughsarrow_forward136 PRACTICAL SPECTROSCOPY Compound 78 is a high-boiling liquid (boiling point 189° C) that contains halogen, but will not react with alkoxides to yield an halogen. ether. The Mass, IR, and 'H NMR spectra, along with 13C NMR data, are given below. Elemental Analysis: C, 35.32; H, 2.47; contains BC Spectral Data: doublet, 137.4 ppm; doublet, 130.1 ppm; doublet, 127.4 ppm; singlet, 97.3 ppm Absorbance Mass Spectrum Intensity 77 77 204 M + 128 40 60 80 100 120 140 160 180 m/e 200 220 280 240 260 300 Infrared Spectrum Wave Number, cm -1 4000 3000 2500 2000 1500 1300 1200 1100 1000 900 800 700 3 6 7 8 9 10 12 13 15 Wavelength, microns 'H NMR wwwww 5 Structure: www ppm, & ©2000 Brooks/Cole Publishing Com-arrow_forward
- no Ai walkthroughsarrow_forward3. Synthesize the following synthon from the indicated starting material. i HO.arrow_forwardIdentifying the stereochemistry of natural Write the complete common (not IUPAC) name of each molecule below. Note: if a molecule is one of a pair of enantiomers, be sure you start its name with D- or L- so we know which enantiomer it is. molecule H O-C-CH2 H3N. HN N H C=O common name (not the IUPAC name) NH3 ☐ H3N H ☐ CH2 Xarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





