
Concept explainers
(a)
Interpretation:
Structural formula of lycopene has to be drawn showing the two sets of four isoprene units linked to form lycopene.
Concept Introduction:
Terpenes are made by joining five-carbon units, usually in a head to tail-fashion.
Isoprene unit:
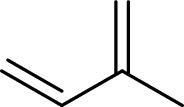
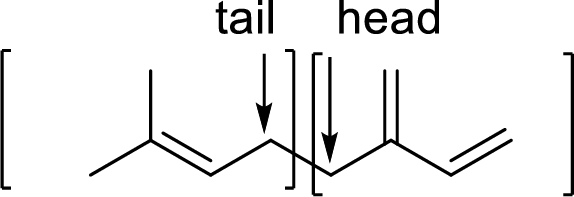
Branched end of isoprene – Head
Unbranched end of isoprene - Tail
(b)
Interpretation:
Number of double in lycopene that has a possibility of cis, trans isomerism has to be given and the double bond that shows cis and that shows trans isomers has to be identified.
Concept Introduction:
Terpenes are made by joining five-carbon units, usually in a head to tail-fashion.
Isoprene unit:
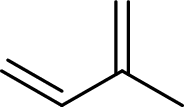
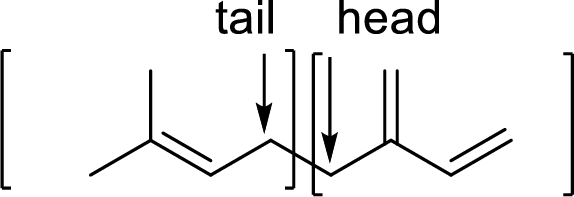
Branched end of isoprene – Head
Unbranched end of isoprene - Tail
Alkene:
- • Trans configuration: In trans configuration, carbon atoms present in the main chain are placed on opposite sides of the carbon-carbon double bond.
- • Cis configuration: In cis configuration, carbon atoms present in the main chain are placed on same sides of the carbon-carbon double bond.
Trending nowThis is a popular solution!

Chapter 5 Solutions
OWLv2 with MindTap Reader, 1 term (6 months) Printed Access Card for Brown/Iverson/Anslyn/Foote's Organic Chemistry, 8th Edition
- Please help me solve this reaction.arrow_forwardIndicate the products obtained by mixing 2,2-dimethylpropanal with acetaldehyde and sodium ethoxide in ethanol.arrow_forwardSynthesize 2-Ethyl-3-methyloxirane from dimethyl(propyl)sulfonium iodide using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Synthesize 2-Hydroxy-2-phenylacetonitrile from phenylmethanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- If possible, please provide the formula of the compound 3,3-dimethylbut-2-enal.arrow_forwardSynthesize 1,4-dibromobenzene from acetanilide (N-phenylacetamide) using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIndicate the products obtained by mixing (3-oxo-3-phenylpropyl)triphenylphosphonium bromide with sodium hydride.arrow_forward
- We mix N-ethyl-2-hexanamine with excess methyl iodide and followed by heating with aqueous Ag2O. Indicate the major products obtained.arrow_forwardIndicate the products obtained by mixing acetophenone with iodine and NaOH.arrow_forwardIndicate the products obtained by mixing 2-Propanone and ethyllithium and performing a subsequent acid hydrolysis.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

