
Concept explainers
(a)
Interpretation:
Atomic orbitals which are used to form each
Concept Introduction:
Hybridization is the mixing of valence atomic orbitals to get equivalent hybridized orbitals that having similar characteristics and energy.
Sigma (σ) bonds are the bonds in which shared hybrid orbital’s electron density are concentrated along the internuclear axis.
Pi (π) bonds are the bonds in which shared unhybridized orbital’s (p, d, etc) electron density are concentrated in above and below of the plane of the molecule.
Geometry of different types of molecule with respect to the hybridizations are mentioned are mentioned below,
(a)
Explanation of Solution
In the marked carbon atom, one s and three p orbital hybridize forming four
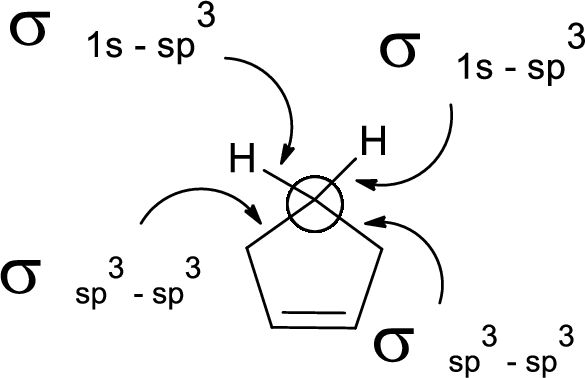
In the marked carbon atom, one s and two p orbital hybridize forming three
(b)
Interpretation:
Atomic orbitals which are used to form each
Concept Introduction:
Hybridization is the mixing of valence atomic orbitals to get equivalent hybridized orbitals that having similar characteristics and energy.
Sigma (σ) bonds are the bonds in which shared hybrid orbital’s electron density are concentrated along the internuclear axis.
Pi (π) bonds are the bonds in which shared unhybridized orbital’s (p, d, etc) electron density are concentrated in above and below of the plane of the molecule.
Geometry of different types of molecule with respect to the hybridizations are mentioned are mentioned below,
(b)
Explanation of Solution
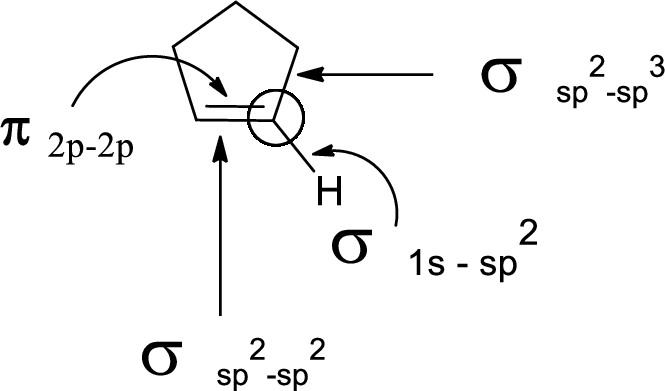
In the marked carbon atom, one s and two p orbital hybridize forming three
(c)
Interpretation:
Atomic orbitals which are used to form each
Concept Introduction:
Hybridization is the mixing of valence atomic orbitals to get equivalent hybridized orbitals that having similar characteristics and energy.
Sigma (σ) bonds are the bonds in which shared hybrid orbital’s electron density are concentrated along the internuclear axis.
Pi (π) bonds are the bonds in which shared unhybridized orbital’s (p, d, etc) electron density are concentrated in above and below of the plane of the molecule.
Geometry of different types of molecule with respect to the hybridizations are mentioned are mentioned below,
(c)
Explanation of Solution
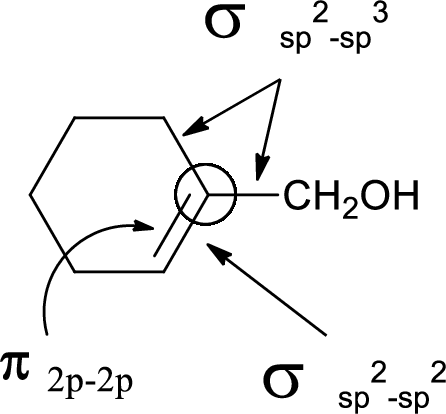
In the marked carbon atom, one s and two p orbital hybridize forming three
(d)
Interpretation:
Atomic orbitals which are used to form each
Concept Introduction:
Hybridization is the mixing of valence atomic orbitals to get equivalent hybridized orbitals that having similar characteristics and energy.
Sigma (σ) bonds are the bonds in which shared hybrid orbital’s electron density are concentrated along the internuclear axis.
Pi (π) bonds are the bonds in which shared unhybridized orbital’s (p, d, etc) electron density are concentrated in above and below of the plane of the molecule.
Geometry of different types of molecule with respect to the hybridizations are mentioned are mentioned below,
(d)
Explanation of Solution
In the marked carbon atom, one s and two p orbital hybridize forming three
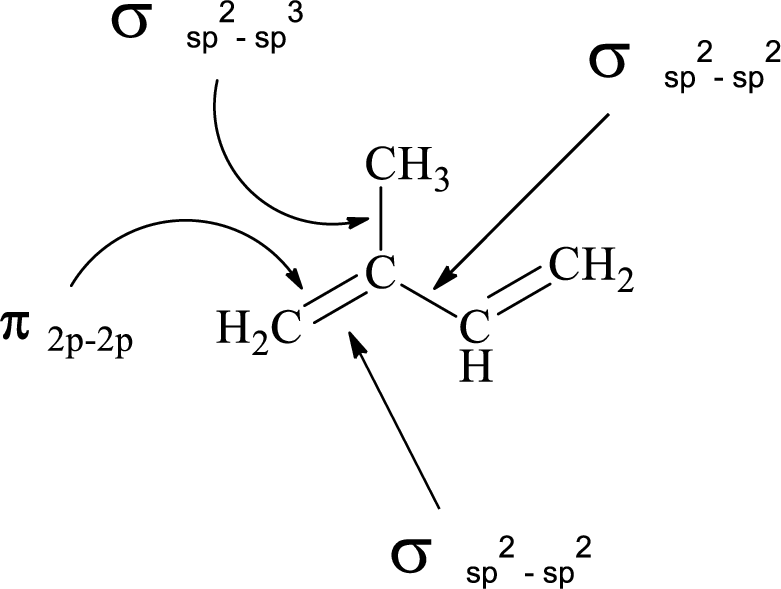
(e)
Interpretation:
Atomic orbitals which are used to form each
Concept Introduction:
Hybridization is the mixing of valence atomic orbitals to get equivalent hybridized orbitals that having similar characteristics and energy.
Sigma (σ) bonds are the bonds in which shared hybrid orbital’s electron density are concentrated along the internuclear axis.
Pi (π) bonds are the bonds in which shared unhybridized orbital’s (p, d, etc) electron density are concentrated in above and below of the plane of the molecule.
Geometry of different types of molecule with respect to the hybridizations are mentioned are mentioned below,
(e)
Explanation of Solution
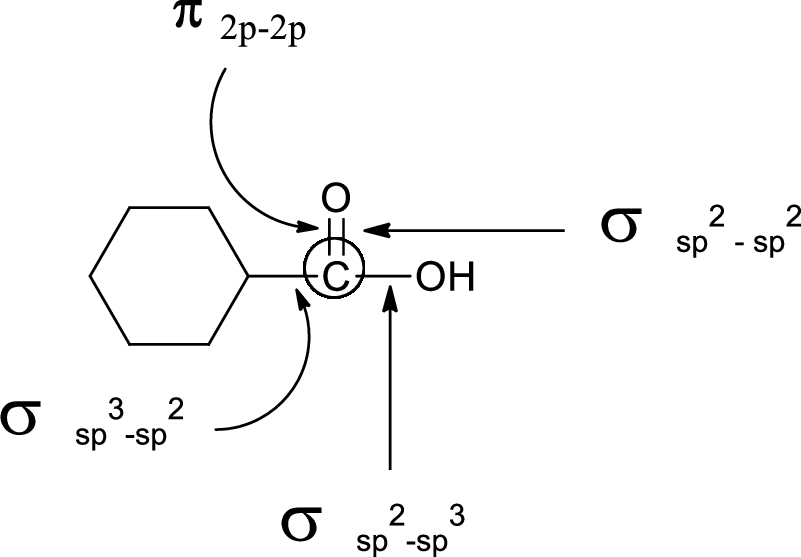
In the marked carbon atom, one s and one p orbital hybridize forming two
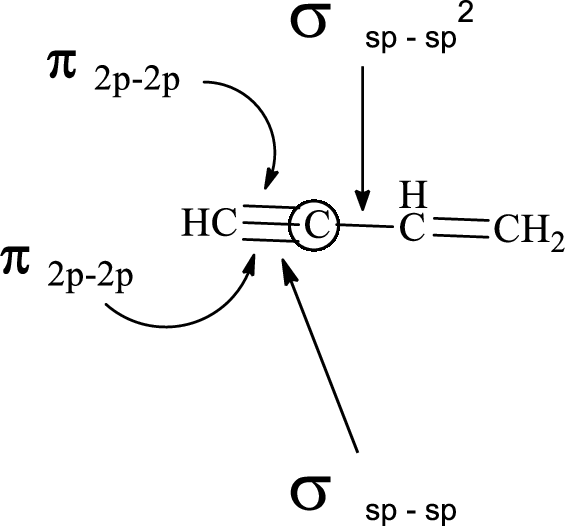
In the marked carbon atom, one s and two p orbital hybridize forming three
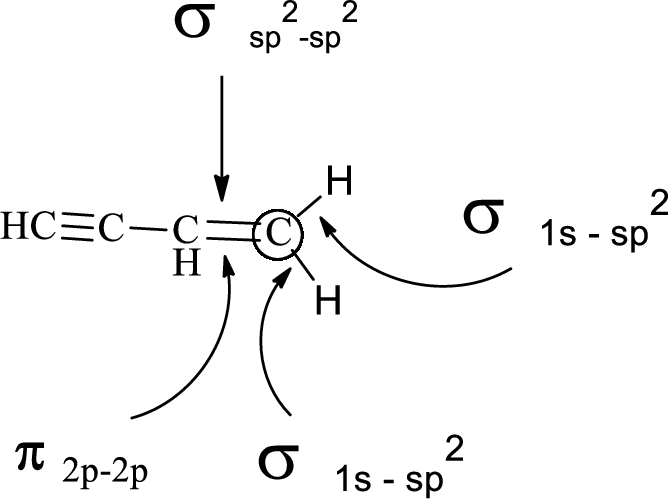
Want to see more full solutions like this?
Chapter 5 Solutions
OWLv2 with MindTap Reader, 1 term (6 months) Printed Access Card for Brown/Iverson/Anslyn/Foote's Organic Chemistry, 8th Edition
- Highlight the chirality (or stereogenic) center(s) in the given compound. A compound may have one or more stereogenic centers. OH OH OH OH OH OHarrow_forwardUsing wedge-and-dash bonds, modify the bonds on the chiral carbon in the molecule below so the molecule has R stereochemical configuration. NH H Br X टेarrow_forwardProvide photos of models of the following molecules. (Include a key for identification of the atoms) 1,2-dichloropropane 2,3,3-trimethylhexane 2-bromo-3-methybutanearrow_forward
- Please draw the structure in the box that is consistent with all the spectral data and alphabetically label all of the equivalent protons in the structure (Ha, Hb, Hc....) in order to assign all the proton NMR peaks. The integrations are computer generated and approximate the number of equivalent protons. Molecular formula: C13H1802 14 13 12 11 10 11 (ppm) Structure with assigned H peaks 2.08 3.13arrow_forwardA 0.10 M solution of acetic acid (CH3COOH, Ka = 1.8 x 10^-5) is titrated with a 0.0250 M solution of magnesium hydroxide (Mg(OH)2). If 10.0 mL of the acid solution is titrated with 10.0 mL of the base solution, what is the pH of the resulting solution?arrow_forwardFirefly luciferin exhibits three rings. Identify which of the rings are aromatic. Identify which lone pairs are involved in establishing aromaticity. The lone pairs are labeled A-D below.arrow_forward
- A 0.10 M solution of acetic acid (CH3COOH, Ka = 1.8 x 10^-5) is titrated with a 0.0250 M solution of magnesium hydroxide (Mg(OH)2). If 10.0 mL of the acid solution is titrated with 10.0 mL of the base solution, what is the pH of the resulting solution?arrow_forwardGiven a complex reaction with rate equation v = k1[A] + k2[A]2, what is the overall reaction order?arrow_forwardPlease draw the structure in the box that is consistent with all the spectral data and alphabetically label all of the equivalent protons in the structure (Ha, Hb, Hc....) in order to assign all the proton NMR peaks. The integrations are computer generated and approximate the number of equivalent protons. Molecular formula: C13H1802 14 13 12 11 10 11 (ppm) Structure with assigned H peaks 2.08 3.13arrow_forward
- CHEMICAL KINETICS. One of the approximation methods for solving the rate equation is the steady-state approximation method. Explain what it consists of.arrow_forwardCHEMICAL KINETICS. One of the approximation methods for solving the rate equation is the limiting or determining step approximation method. Explain what it consists of.arrow_forwardCHEMICAL KINETICS. Indicate the approximation methods for solving the rate equation.arrow_forward
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax


